From a scientific and medical perspective, bioterrorism—using biological weapons to produce disease in humans—can be viewed as a variation of the problem of emerging infectious diseases, the only difference being that increased virulence or intentional release are deliberate acts. The United States public health system and primary healthcare providers must be prepared to address various biological agents, including pathogens that are rarely seen in this country.
Covert vs. Overt Bioterrorism
As with chemical agents, the intentional release of biological agents can be either covert or overt. A covert release is unannounced and hidden, and may go unnoticed for days or even weeks. The presence of ill individuals may be the first sign of a release, and those infected may have inadvertently infected others. An infected person may seek medical care anywhere within the healthcare system, possibly at a distance from the release area.
An overt release is immediately apparent and may even be announced. In an overt release, the healthcare system and public health officials may be overwhelmed by requests for information and treatment. Hospitals, clinics, emergency responders, and communication systems will be pressed into immediate service. An overt release has the potential to cause widespread panic.
Whether the release is covert or overt, healthcare providers should be alert to illness patterns and diagnostic clues that indicate an unusual infectious disease outbreak that could be associated with intentional release of a biological agent. In addition that should watch for increases in unexpected or unexplained illnesses and know how to activate the public health response system if an outbreak is suspected (CDC, 2001). Well-trained and educated first responders, first receivers, and public health personnel are essential to an organized and successful response.
Improving Response to Biologically Induced Illness
Healthcare providers, clinical laboratory personnel, infection control professionals, and public health departments play critical and complementary roles in the recognition and response to illness caused by the intentional release of biological agents. Syndrome descriptions, epidemiologic clues, and laboratory recommendations provide basic guidance that can improve recognition of these events (CDC, 2001).
Since 9/11, state and local health departments have initiated activities to improve recognition, reporting, and response, ranging from enhancing communications to conducting special surveillance projects. This includes active tracking for changes in the number of hospital admissions, emergency department visits, and occurrence of specific syndromes. Bioterrorism preparedness activities and work with emerging infectious diseases have helped public health agencies prepare for the intentional release of a biological agent (CDC, 2001). The CDC’s Emergency Preparedness and Response website has links to and information on the various tools available, as well as other resources.
Recognizing Clinical Syndromes
Work continues on syndromic surveillance projects and the CDC maintains current data on this research. The term syndromic surveillance means watching for health-related data that signal sufficient probability of a case or an outbreak to warrant further public health response. Historically, syndromic surveillance was used in investigating potential cases, but its utility for detecting outbreaks associated with bioterrorism is increasingly being explored by public health officials. Technology changes and the plethora of programs and data have also affected these efforts (CDC, 2004b, 2012; Dembek, 2004). (See also the CDC resource website).
The release of a biological agent may not have an immediate impact because of the delay between exposure and onset of illness, and because outbreaks associated with intentional releases may resemble naturally occurring ones. Nevertheless, healthcare workers should be familiar with indications of intentional release of a biological agent and know when, and to whom, to report a suspected outbreak.
These indications include unusual clustering of illness, patients presenting with clinical signs and symptoms that suggest an infectious disease outbreak, unusual age distribution for common diseases, and a large number of cases of acute flaccid paralysis with prominent bulbar palsies, which is suggestive of a release of botulinum toxin (CDC, 2001).
Epidemiologic Clues That May Signal a Covert Bioterrorism Attack
- Large number of ill persons with similar disease or syndrome.
- Large number of unexplained disease, syndrome or deaths.
- Unusual illness in a population.
- Higher morbidity and mortality than expected with a common disease or syndrome.
- Failure of a common disease to respond to usual therapy.
- Single case of disease caused by an uncommon agent.
- Multiple unusual or unexplained disease entities coexisting in the same patient without other explanation.
- Disease with an unusual geographic or seasonal distribution.
- Multiple atypical presentations of disease agents.
- Similar genetic type among agents isolated from temporally or spatially distinct sources.
- Unusual, atypical, genetically engineered, or antiquated strain of agent.
- Endemic disease with unexplained increase in incidence.
- Simultaneous clusters of similar illness in non-contiguous areas, domestic or foreign.
- Atypical aerosol, food, or water transmission.
- Ill people presenting near the same time.
- Deaths or illness among animals that precedes or accompanies illness or death in humans.
- No illness in people not exposed to common ventilation systems, but illness among those people in proximity to the systems (CDC, 2001a)
As noted earlier, a variety of factors affect the potential public health impact of an intentionally released biological agent:
- Lethality—how effectively it kills
- Infectivity—how easily it spreads
- Virulence—how likely it is to cause disease
- How easily is it dispersed
- Availability of medical treatment and/or vaccine
- Dosage needed to cause disease
- Stability of the compound (NTI, 2015a)
It may be difficult to pinpoint the time and location of a biological agent’s release because of the variation in incubation period among organisms. Some diseases show a rapid onset of symptoms and early treatment is critical. For example, plague has a rapid onset and is potentially fatal within 12 to 24 hours if untreated; botulism toxin also has a rapid onset and requires immediate supportive treatment. On the other hand, smallpox can be treated effectively by vaccination within 2 to 3 days of symptom onset. But smallpox, like plague, is highly contagious and has the potential to cause widespread panic, and in the case of smallpox, which is believed to have been eradicated, not enough vaccine exists should a widespread outbreak occur. Conversely, plague and anthrax, despite their potential for causing serious illness and death, are effectively treated with antibiotics.
Categories of Diseases and Biological Agents
Bioterrorism agents can be separated into three categories, depending on how easily they can be spread and the severity of illness or death they cause. Category A agents are considered the highest risk and Category C agents are those that are considered emerging threats for disease (CDC, 2007).
Category A Diseases or Agents
Category A diseases or agents are high priority and include organisms that pose the highest risk to the public and national security because they:
- Are easily spread or transmitted from person to person
- Result in high mortality rates and have the potential for major public health impact
- May cause public panic and social disruption
- Require special action for public health preparedness (CDC, 2007)

A letter sent in 2001 to Senate Majority Leader Tom Daschle contained anthrax powder. Beginning one week after the September 11 attacks, letters containing anthrax spores were mailed to several news media offices and two U.S. Senators, killing five people and infecting 17 others. Source: Wikimedia Commons.
Category A bioterrorism agents are:
- Anthrax
- Botulism
- Plague
- Smallpox
- Tularemia
- Viral hemorrhagic fevers (VHF) (CDC, 2013a)
Category B Diseases or Agents
Category B diseases or agents are the second highest priority because they:
- Are moderately easy to disseminate
- Result in moderate illness rates and low mortality rates
- Require specific enhancements of CDC’s laboratory capacity and enhanced disease surveillance (CDC, 2007)
Category B diseases or agents include:
- Brucellosis (Brucella species)
- Epsilon toxin of Clostridium perfringens
- Food safety threats (Salmonella species, Escherichia coli O157:H7, and Shigella)
- Glanders (Burkholderia mallei)
- Melioidosis (Burkholderia pseudomallei)
- Psittacosis (Chlamydia psittaci)
- Q fever (Coxiella burnetii)
- Ricin toxin from Ricinus communis (castor beans)
- Staphylococcal enterotoxin B
- Typhus fever (Rickettsia prowazekii)
- Viral encephalitis (alphaviruses—Venezuelan equine encephalitis, eastern and western equine encephalitis)
- Water safety threats (Vibrio cholerae, Cryptosporidium parvum) (CDC, 2008)
Category C Diseases or Agents
Category C diseases or agents are the third highest priority and include emerging pathogens that could be engineered for mass dissemination in the future because:
- Are easily available
- Are easily produced and spread
- Have potential for high morbidity and mortality rates and major health impact (CDC, 2007)
Clinical Features of High-Priority Agents
Four category A diseases have been the focus of the CDC’s efforts to educate the healthcare community about bioterrorism potential: anthrax, botulism, plague, and smallpox. The CDC does not prioritize these agents in any order of importance or likelihood of use. Other agents with bioterrorism potential include those that cause tularemia and viral hemorrhagic fevers (category A), brucellosis, Q fever, viral encephalitis, and disease associated with staphylococcal enterotoxin, category B. Other important category B agents include any organism that threatens the water or food supply.
Anthrax
Anthrax has been recognized as an infectious disease of animals and humans for millennia. Within the United States, animal anthrax is reported in most years, but naturally occurring human anthrax is rare. Worldwide, however, the disease is common in wild and domestic animals and not uncommon among persons who interact with animals in agricultural regions of South and Central America, sub-Saharan Africa, central and southwestern Asia, and southern and eastern Europe (Hendricks, et al. [CDC], 2014).
Bacillus anthracis, the causative agent of anthrax, is a nonmotile spore-forming, gram-positive, rod-shaped bacterium. Biodefense experts often place B. anthracis at or near the top of the list for potential threat agents. Inhalation anthrax is particularly deadly, as demonstrated by the 1979 accidental release of B. anthracis from a military microbiology facility in the Sverdlovsk region of Russia; 88% (66/75) of patients reported with inhalation anthrax died. More recently, humans have acquired disease from exposure to spores released purposefully as a bioterrorist weapon and accidentally from naturally occurring sources (Hendricks, et al. [CDC], 2014).
If a bioterrorist attack were to happen, Bacillus anthracis would be one of the biological agents most likely to be used. Biological agents are germs that can sicken or kill people, livestock, or crops. Anthrax is one of the most likely agents to be used because:
- Anthrax spores are easily found in nature, can be produced in a lab, and can last for a long time in the environment.
- Anthrax makes a good weapon because it can be released quietly and without anyone knowing. The microscopic spores could be put into powders, sprays, food, and water. Because they are so small, you may not be able to see, smell, or taste them.
- Anthrax has been used as a weapon before. (CDC, 2014)
Anthrax has been used as a weapon around the world for nearly a century. In 2001, powdered anthrax spores were deliberately put into letters that were mailed through the U.S. postal system. Twenty-two people, including 12 mail handlers, got anthrax, and five of these 22 people died.
A subset of select agents and toxins have been designated as Tier 1 because these biological agents and toxins present the greatest risk of deliberate misuse with significant potential for mass casualties or devastating effect to the economy, critical infrastructure, or public confidence, and pose a severe threat to public health and safety. Bacillus anthracis is a Tier 1 agent.
An anthrax attack could take many forms. For example, it could be placed in letters and mailed, as was done in 2001, or it could be put into food or water. Anthrax also could be released into the air from a truck, building, or plane. This type of attack would mean the anthrax spores could easily be blown around by the wind or carried on people’s clothes, shoes, and other objects. It only takes a small amount of anthrax to infect a large number of people.
If anthrax spores were released into the air, people could breathe them in and get sick with anthrax. Inhalation anthrax is the most serious form and can kill quickly if not treated immediately. If the attack were not detected by one of the monitoring systems in place in the United States, it might go unnoticed until doctors begin to see unusual patterns of illness among sick people showing up at emergency rooms (CDC, 2014).
There are four clinical forms of anthrax: cutaneous or skin, respiratory tract or inhalation, gastrointestinal, and injection anthrax (has occurred in Europe but not in the US) (CDC, 2013b).
Cutaneous Anthrax
When anthrax spores get into the skin, usually through a cut or scrape, a person can develop cutaneous anthrax. This can happen when a person handles infected animals or contaminated animal products like wool, hides, or hair. Cutaneous anthrax is most common on the head, neck, forearms, and hands. It affects the skin and tissue around the site of infection.
Cutaneous anthrax is the most common form of anthrax infection, and it is also considered to be the least dangerous. Infection usually develops from 1 to 7 days after exposure. Without treatment, up to 20% of people with cutaneous anthrax may die. However, with proper treatment, almost all patients with cutaneous anthrax survive (CDC, 2013b).
Cutaneous anthrax symptoms can include:
- A group of small blisters or bumps that may itch
- A painless skin sore (ulcer) with a black center that appears after the small blisters or bumps
- Most often the sore will be on the face, neck, arms, or hands
- Swelling can occur around the sore (CDC, 2013b)
Inhalation Anthrax
When a person breathes in anthrax spores, they can develop inhalation anthrax. People who work in places such as wool mills, slaughterhouses, and tanneries may breathe in the spores when working with infected animals or contaminated animal products from infected animals. Inhalation anthrax starts primarily in the lymph nodes in the chest before spreading throughout the rest of the body, ultimately causing severe breathing problems and shock.
Inhalation anthrax is considered to be the most deadly form of anthrax. Infection usually develops within a week after exposure, but it can take up to 2 months. Without treatment, only about 10% to 15% of patients with inhalation anthrax survive. However, with aggressive treatment, about 55% of patients survive (CDC, 2013b).
Inhalation anthrax symptoms can include:
- Fever and chills
- Chest discomfort
- Shortness of breath
- Confusion or dizziness
- Cough
- Nausea, vomiting, or stomach pains
- Headache
- Sweats (often drenching)
- Extreme tiredness
- Body aches (CDC, 2013b)
Gastrointestinal Anthrax
When a person eats raw or undercooked meat from an animal infected with anthrax, they can develop gastrointestinal anthrax. Once ingested, anthrax spores can affect the upper gastrointestinal tract (throat and esophagus), stomach, and intestines.
Gastrointestinal anthrax has rarely been reported in the United States. Infection usually develops from 1 to 7 days after exposure. Without treatment, more than half of patients with gastrointestinal anthrax die. However, with proper treatment, 60% of patients survive (CDC, 2013b).
Gastrointestinal anthrax symptoms can include:
- Fever and chills
- Swelling of neck or neck glands
- Sore throat
- Painful swallowing
- Hoarseness
- Nausea and vomiting, especially bloody vomiting
- Diarrhea or bloody diarrhea
- Headache
- Flushing (red face) and red eyes
- Stomach pain
- Fainting
- Swelling of abdomen (stomach) (CDC, 2013b)
Injection Anthrax
Recently, another type of anthrax infection has been identified in heroin-injecting drug users in northern Europe. This type of infection has never been reported in the United States.
Symptoms may be similar to those of cutaneous anthrax, but there may be infection deep under the skin or in the muscle where the drug was injected. Injection anthrax can spread throughout the body faster and be harder to recognize and treat. Lots of other more common bacteria can cause skin and injection site infections, so a skin or injection site infection in a drug user does not necessarily mean the person has anthrax (CDC, 2013b).
Injection anthrax symptoms can include:
- Fever and chills
- A group of small blisters or bumps that may itch, appearing where the drug was injected
- A painless skin sore with a black center that appears after the blisters or bumps
- Swelling around the sore
- Abscesses deep under the skin or in the muscle where the drug was injected
- Keep in mind
- Symptoms are similar to those of cutaneous anthrax, but injection anthrax can spread throughout the body faster and be harder to recognize and treat than cutaneous anthrax.
- Skin and injection site infections associated with injection drug use are common and do not necessarily mean the person has anthrax. (CDC, 2013b)
Botulism
Botulism is a neuroparalytic (muscle-paralyzing) disease whose agent is the toxin produced by Clostridium botulinum—an encapsulated, anaerobe, gram-positive, spore-forming, rod-shaped bacterium (CDC, 2006). Botulism neurotoxin is an extremely potent organism; less than 1 microgram causes fatality in adults. It causes paralysis by inhibiting the release of acetylcholine at the neuromuscular junction; respiratory paralysis and death result if left untreated. There are four forms of naturally occurring botulism:
Foodborne Botulism
- Caused by ingestion of pre-formed toxin
Infant Botulism
- Caused by ingestion of C. botulinum, which produces toxin in the intestinal tract
Wound Botulism
- Caused by wound infection with C. botulinum that secretes the toxin
Adult Intestinal Colonization
- Rare, caused when C. botulinum colonizes the intestinal tract of children or adults, usually with gastrointestinal abnormalities (CDC, 2006)
Botulinum toxin as a biological weapon
- Aerosolized botulinum toxin is a possible mechanism for a bioterrorism attack
- Inhalational botulism does not occur naturally
- Inhalational botulism cannot be clinically differentiated from the three naturally occurring forms
- Indications of intentional release of a biologic agent may include:
- An unusual geographic clustering of illness (eg, persons who attended the same public event or gathering)
- A large number of cases of acute flaccid paralysis with prominent bulbar palsies, especially if occurring in otherwise healthy persons (CDC, 2006)
Botulism is not transmissible from person to person. For foodborne botulism, symptoms begin within 6 hours to 10 days after exposure (often within 12–36 hours), and could be shorter in inhalational botulism (CDC, 2006b).
|
Symptoms, Diagnosis, and Treatment of Botulism |
|
|---|---|
|
Symptoms/ |
|
|
Diagnosis/ |
|
|
Treatment |
|
|
Prophylaxis |
|
Plague (Yersinia pestis)
Plague is an acute and potentially fatal bacterial infection that affects humans and animals and is caused by Y. pestis. Plague usually presents as 1 of 5 principal clinical syndromes: bubonic, pneumonic, septicemic, plague meningitis, or pharyngeal. Plague is a naturally occurring disease that has been endemic in the United States since 1900. Approximately 5 to 15 cases occur per year, with the greatest concentration of cases in Arizona, Colorado, and New Mexico (CDC, 2004a).
An immediate and coordinated public health and medical response would be required in the event of the intentional use of plague. Therefore, any case of plague should be reported to the state health department immediately. Reporting is especially important when a case of plague occurs outside of a typically affected area (CDC, 2004a).
With bubonic plague, the infection is transmitted by the bite of an infected flea or exposure to infected material through a break in the skin. Bubonic plague cannot be transmitted from person to person. If bubonic plague is not treated, the bacteria can spread through the bloodstream and infect the lungs, causing a secondary infection of pneumonic or septicemic plague (CDC, 2004a).
Pneumonic plague is a pulmonary infection that occurs upon inhalation of plague bacteria. Pneumonic plague can be transmitted person to person through respiratory droplets with direct close contact, and without early treatment in less than 24 hours, pneumonic plague almost universally leads to respiratory failure, shock, and rapid death (CDC, 2004a).
Infection via inhalation of infective respiratory droplets or aerosols is rare with naturally occurring plague in the United States, but is the most likely route of transmission in a bioterrorist event. If Y. pestis were to be used as a bioweapon, it would be most dangerous if released as an aerosol. An aerosol release would be expected to result in an outbreak of the pneumonic form of plague and it may also cause the less common pharyngeal plague and ocular plague (CDC, 2004a).
The primary form of septicemic plague results from direct inoculation and multiplication of plague bacilli in the bloodstream, while the secondary form is a development of untreated pneumonic or bubonic plague (CDC, 2004a).
Bubonic Plague
- Incubation period: 2 to 6 days
- Symptoms
- Lymphadenopathy and fever are both early symptoms of bubonic plague.
- Patients develop buboes, which are grossly enlarged, extremely tender lymph nodes draining at the respective site of inoculation.
- Progression of disease: If bubonic plague is not treated, the bacteria can spread through the bloodstream, causing septicemia, or it can infect the lungs, causing a secondary case of pneumonic plague. Rarely, it can progress to meningitis. (CDC, 2004a)
Pneumonic Plague
- Incubation period: 2 to 4 days with range of 1 to 6 days
- Symptoms
- Acute onset of fever, chills, malaise, and myalgias associated with progressive lethargy
- A productive cough of copious watery mucoid sputum that may be bloody
- Associated chest pain and increasing dyspnea
- Progression of disease: As the disease progresses, adult respiratory distress syndrome (ARDS) characterized by refractory pulmonary edema may occur. Signs of shock, including hypotension and eventual multi-organ failure, may also occur. Without early detection and treatment in less than 24 hours, pneumonic plague is almost universally fatal. (CDC, 2004a)
Septicemic Plague
- Incubation period: Occurs when plague bacteria multiply in the blood. Most commonly, septicemic plague presents as a complication of pneumonic or bubonic plague, but primary septicemic plague can occur.
- Symptoms: Acute onset of fever, chills, prostration, abdominal pain, nausea, and vomiting.
- Progression of disease: As the disease progresses, purpura may develop, as well as possible disseminated intravascular coagulation (DIC). Eventually, hypotension and other signs of shock appear. Septicemic plague is often fatal even when treated. (CDC, 2004a)
Smallpox (Variola)
Smallpox is a serious, contagious, and sometimes fatal infectious disease. There is no specific treatment for smallpox disease, and the only prevention is vaccination. The pox part of smallpox is derived from the Latin word for “spotted” and refers to the raised bumps that appear on the face and body of an infected person (CDC, 2004c).
There are two clinical forms of smallpox. Variola major is the severe and most common form of smallpox, with a more extensive rash and higher fever. There are four types of variola major smallpox: ordinary (the most frequent type, accounting for 90% or more of cases); modified (mild and occurring in previously vaccinated persons); flat; and hemorrhagic (both rare and very severe). Historically, variola major has an overall fatality rate of about 30%; however, flat and hemorrhagic smallpox usually are fatal. Variola minor is a less common presentation of smallpox, and a much less severe disease, with death rates historically of 1% or less (CDC, 2004c).
Smallpox is caused by the variola virus, which emerged in human populations thousands of years ago, but the disease is now eradicated after a successful worldwide vaccination program. After the disease was eliminated from the world, routine vaccination against smallpox among the general public was stopped because it was no longer necessary for prevention. Except for laboratory stockpiles, the variola virus has been eliminated. However, in the aftermath of 9/11, there is heightened concern that the variola virus might be used as an agent of bioterrorism. For this reason, the U.S. government is taking precautions for dealing with a smallpox outbreak (CDC, 2004c).
Generally, direct and fairly prolonged face-to-face contact is required to spread smallpox from one person to another. Smallpox also can be spread through direct contact with infected bodily fluids or contaminated objects such as bedding or clothing. Rarely, smallpox has been spread by virus carried in the air in enclosed settings such as buildings, buses, and trains. Humans are the only natural hosts of variola. Smallpox is not known to be transmitted by insects or animals.
A person with smallpox is sometimes contagious with onset of fever (prodrome phase), but the person becomes most contagious with the onset of rash. At this stage the infected person is usually very sick and not able to move around in the community. The infected person is contagious until the last smallpox scab falls off (CDC, 2004c).
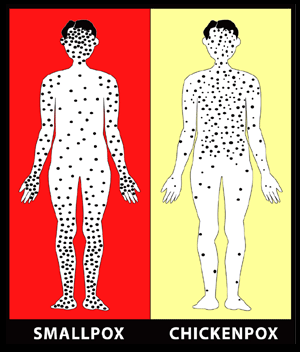
Source: CDC.
The acute clinical symptoms of smallpox resemble other acute viral illnesses, such as influenza, beginning with a 2- to 4-day nonspecific prodrome of fever and myalgias before rash onset. Several clinical features can help clinicians differentiate varicella (chickenpox) from smallpox. The rash of varicella is most prominent on the trunk and develops in successive groups of lesions over several days, resulting in lesions in various stages of development and resolution. In comparison, the vesicular/pustular rash of smallpox is typically most prominent on the face and extremities, and lesions develop at the same time (CDC, 2001; CDC, 2004c).
The only weapons against smallpox are vaccination and patient isolation. Those caring for a patient with smallpox should wear an N95 mask and follow airborne and contact isolation precautions. HEPA filters do remove smallpox virus, but proper procedures must be followed for their effective use. Vaccination before exposure, or within 3 days after exposure, affords almost complete protection against the disease. Vaccination as late as 4 to 7 days after exposure likely offers some protection from disease or may modify the severity of disease (CDC, 2009a,b).
The smallpox vaccine is made from a virus called vaccinia, which is a pox-type virus related to smallpox. The vaccine contains the live vaccinia virus (other vaccines containing live virus include measles, mumps, and German measles) and for that reason the vaccination site must be treated carefully to prevent the virus from spreading. The vaccine can have side effects; however, it does not contain the smallpox virus and cannot give you smallpox (CDC, 2009c).
Best Practices for First Receivers
Healthcare workers risk occupational exposures to biological materials when a hospital receives contaminated patients, particularly during mass casualty events. Hospital employees termed first receivers work at a site removed from where the hazardous release occurred. This means that their exposures are limited to the substances transported to the hospital on the skin, hair, clothing, or personal effects of the victims. The location and limited source of contaminants distinguishes first receivers from first responders such as firefighters, law enforcement, and ambulance service personnel, who typically respond to the incident site (OSHA, 2005).
Worst-case scenarios take into account challenges associated with communication, resources, and victims. During mass-casualty emergencies, hospitals can anticipate little or no warning before victims begin arriving. First receivers can anticipate that information regarding the hazardous agents may not be available immediately. Hospitals can also anticipate a large number of self-referred victims (as many as 80% of the total number of victims) and should assume victims will not have been decontaminated prior to arriving at the hospital (OSHA, 2005).
An employee’s role at a facility and the corresponding hazards the employee might encounter dictate the level of training that must be provided to any individual first receiver. Selection of personal protective equipment (PPE) must be based on a hazard assessment that carefully considers both of these factors, along with the steps taken to minimize the extent of the employee’s contact with hazardous substances (OSHA, 2005). Surge capacity, triage, decontamination, security, and disposal of contaminated wastewater must also be addressed.
Surge Capacity
In the event of a mass casualty event, healthcare organizations must be able to increase their services quickly in response to the crisis. This is an organization’s surge capacity, “the ability to expand care capabilities in response to sudden or prolonged demand” (JCAHO, 2003; Kelen, 2008). Staffing levels, education and training, decontamination capabilities, vaccination programs for direct caregivers, volunteer resources, and stockpiling of supplies must be assessed while, in most cases, routine care continues.
Individual personnel on an emergency response team have slightly differing concerns and responsibilities when it comes to surge situations. While surge capacity planning is an administrative level concern, individual healthcare providers should understand the basic concept and the need for guidelines in order to participate effectively in training and any necessary implementation. The CDC’s handbook, Updated In a Moment’s Notice: Surge Capacity in Terrorist Bombings, and other good general resources are available at this website: http://stacks.cdc.gov/view/cdc/5713/ (CDC, 2010).
The ability of the organization to “degrade gracefully” must also be considered. A healthcare organization should have a plan to deal with a reduction in services as the number of patients increases. The goal is to engineer and manage failures and thus to avoid “catastrophic failure” (JCAHO, 2003). During a state of emergency, it may be impossible to follow normal practice guidelines. The Joint Commission recommends that hospitals and oversight agencies “provide for waiver of regulatory requirements under conditions of extreme emergency” (JCAHO, 2003).
Triage
Pre-decontamination triage serves three purposes:
- Distinguishes contaminated individuals from other patients arriving at the hospital by identifying symptoms and victim’s proximity to a known chemical release
- Identifies patients who require immediate stabilization before they enter the decontamination system
- Identifies injuries or critical pre-hospital treatment materials that will require special handling inside the decontamination system (OSHA, 2005)
Post decontamination triage for medical treatment should occur in the hospital post-decontamination zone after victims are inspected and found to be free of contamination. Some hospitals combine decontamination and initial medical treatment (such as antidotes), which means either the healthcare worker attempts medical triage while wearing PPE (preferred) or the worker is at risk of exposure from victims who have not been adequately decontaminated (OSHA, 2005).
Decontamination Activities
Hospitals must identify spaces that will support decontamination activities (including equipment storage) and ensure that operations can continue in the event that one area of the hospital becomes contaminated. Hospitals planning additions or remodeling projects have a unique opportunity to design spaces appropriately. Other hospitals should use creative planning to identify existing architectural features that they can use to their advantage. Nonambulatory victims can require a substantial proportion of first receivers’ time and efforts, and first receivers are likely to experience the greatest exposure while assisting these victims (OSHA, 2005).
If decontamination is necessary, numerous agencies and organizations recommend a shower time of approximately five minutes for contaminated victims brought to a hospital. Despite the fact that there is no empirical data, operational procedures deem this time to be adequate. Numerous agencies and programs recommend the use of water and a liquid soap with good surfactant properties (such as hand dishwashing detergent) to decontaminate victims during emergencies and for mass casualties involving hazardous substances (OSHA, 2005).
Isolation and Lockdown
Hospitals can use a variety of methods to limit unauthorized access to the emergency department until the victims have been decontaminated. The methods range from a guard at the locked door to sophisticated keycard systems controlled at a central command center. These more complex systems tend to be associated with urban or recently modernized hospitals and are intended for use in any type of disturbance. Hospitals can use these methods if situations suggest that an unruly crowd will force its way into the hospital (OSHA, 2005).
Security
Site security helps maintain order and control traffic around the decontamination facility and the hospital entrances. Security officers might need to control a contaminated individual to prevent other staff from becoming exposed and to protect equipment. Security officers also ensure contaminated victims do not bypass the decontamination hospital or enter the ED without passing inspection. In cases of civil disturbance, properly identified security officers protect the decontamination facility and staff so normal operations can continue (OSHA, 2005).
Personal Protective Equipment
Hospitals should select personal protective equipment (PPE) such as respirators, suits, gloves, and face and eye protection based on a hazard assessment that identifies the hazards to which employees might be exposed. Under OSHA’s Personal Protective Equipment Standard, or the parallel State Plan standards, all employers, including hospitals, must certify in writing that the hazard assessment has been performed. For first-receiver PPE, hospitals may base the hazard assessment on OSHA’s Best Practices document. Hospitals likely to respond to incidents involving a specific hazard should adjust the PPE accordingly (OSHA, 2005).
OSHA’s Personal Protective Equipment Standard also requires that employees be provided with equipment that fits appropriately. Some hospitals assign a set of protective equipment to a specific individual, and that equipment is stored in a container marked with the individual’s name. Other hospitals maintain general supplies of PPE, storing sets of equipment by size. In this case, the packages are clearly marked only with the size. Each first receiver tries on equipment in advance to determine what size group fits best so that, during an emergency, the employee can quickly locate an appropriate PPE set (OSHA, 2005).
Personal protective equipment selection for first receivers has been a topic of extensive discussion. At the root of this discussion is the need for hospitals to provide adequate protection for the reasonably anticipated worst-case scenario, despite having limited information regarding the nature of the substance with which victims may be contaminated. This lack of information challenges hospitals’ abilities to conduct the hazard assessments on which PPE selection must be based (OSHA, 2005).
Infection Control
Heightened awareness by infection control (IC) professionals facilitates recognition of the release of a biological agent. Infection control professionals are involved with many aspects of hospital operations and several departments, and with their counterparts in other hospitals. As a result, they may recognize changing patterns or clusters in a hospital or in a community that might otherwise go unrecognized (CDC, 2001).
Infection control professionals should ensure that hospitals have current telephone numbers for notification of both internal and external contacts and that they are distributed to the appropriate personnel. They should work with clinical microbiology laboratories, on- or off-site, that receive specimens for testing from their facility to ensure that cultures from suspicious cases are evaluated appropriately (CDC, 2001).
Wastewater Management
Wastewater from decontamination showers can contain low-level concentrations of the substance(s) with which victims are contaminated. Given the opportunity to plan for decontamination activities (by designing and installing or purchasing decontamination facilities, developing procedures, and preparing staff), hospitals should consider the management of decontamination shower water as part of their emergency preparedness plan (OSHA, 2005).
Decontaminating Surfaces and Equipment
The hospital emergency management plan should include procedures for cleaning equipment and surfaces during and after an incident. Cleaning should be performed by employees who are properly protected and trained. Items that cannot be decontaminated safely should be processed for appropriate disposal. It is unlikely that portable gear could be adequately decontaminated after an incident involving a persistent or highly toxic agent (OSHA, 2005).
Reporting an Incident of Bioterrorism
In the event that an incident of bioterrorism occurs in your community, you should know what to report and to whom the report should be sent. First reporters should start at the healthcare organization or hospital level by reporting to the department supervisor, laboratory, and infection control department. Then contact the local health/regional departments, which will contact your state’s health department and the CDC. Successful reporting of a bioterrorism event results from good planning, education, and awareness, as well as regular standardized testing before an occurrence.
In most cases telephone will still be the primary means for immediate reporting because it is direct, rapid, and easy-to-use. There should always be a backup communication plan (eg, cell phones or other means) in case of a telephone system failure. In every institution standards should be established to ensure a reliable and immediate response to notifiable diseases and health conditions.
Back Next