The only way to know your HIV status is to get tested. It is recommended that everyone between the ages of 13 and 64 for get tested at least once. Those who have put themselves at risk through anal, vaginal, or oral sex, or shared needles, as well as anyone who has had an occupational exposure, should be tested. Partners of people with risk factors, (along with their partners) should consider testing.
Early detection allows people with HIV to receive medical treatment soon after infection. Early treatment protects the immune system of infected people and encourages healthy habits and safer sex practices. New drug therapies can sustain an infected person's health for long periods of time and early detection allows people to take precautions to prevent infecting others.
Many HIV tests are now quick, free, and painless. Ask your healthcare provider for an HIV test or use the HIV Services Locator to find a testing site near you. You can also buy an FDA-approved home testing kit at a pharmacy or online. Source: HIV.gov.
There currently is no test that can detect HIV immediately after exposure as the viral load may be too small to detect. If someone believes they have been exposed to HIV they should begin post exposure prophylaxis (PEP) medication as soon as possible. PEP is the use of antiretroviral drugs after a single high-risk event to stop HIV seroconversion. PEP must be started as soon as possible to be effective—and always within 72 hours of a possible exposure.
Florida has more than 1,460 publicly funded and registered testing sites, with 70% using rapid tests. A new, fourth-generation screening and confirmatory test has replaced the traditional Western Blot, allowing quicker results and faster initiation of treatment (FDOH, 2021, July 16).
Florida law requires those who perform HIV tests in county health departments and other registered testing sites to obtain the informed consent of the test subject, confirm positive preliminary results with a supplemental test before informing the test subject of the result (except as provided for by statute), and make a reasonable attempt to notify the test subject of the test result (FDOH, 2021, July 16).
Source: CDC.
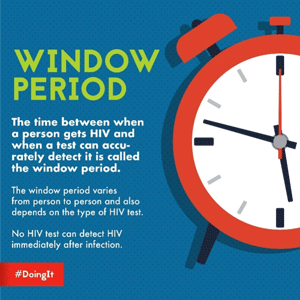
Each type of HIV test has a “window period” that varies from person to person and depends on the type of test. The window period is the time between infection with the virus and when the HIV-infected person develops enough antibodies to be detected by the antibody test. Until the infected person’s immune system makes enough antibodies to be detected, the test will be negative even though the person is infected with HIV.
There is no way to know how long an infected person will take to develop antibodies. Some infected people can produce antibodies as early as 2 weeks after infection and almost everyone will develop enough antibodies to be detected by 12 weeks (3 months) after infection.
Knowing your HIV status gives you powerful information to help you take steps to keep you and your partner(s) healthy. Source: HIV.gov.
Because people who are newly infected have so few antibodies to fight HIV, the virus can quickly grow and multiply. During this time, they can have a large amount of virus in their blood, making them highly infectious. During the window period it is possible for an infected person to test negative but still be able to infect another person.
HIV screening is recommended for patients in all healthcare settings following notification unless the patient declines (opt-out screening). People at high risk for HIV infection should be screened for HIV at least annually (CDC, 2019, October 21).
For pregnant women, HIV screening tests:
- Should be included in the routine panel of prenatal screening tests for all pregnant women.
- Is recommended after the patient is notified that testing will be performed unless the patient declines (opt-out screening).
Separate written consent for HIV testing should not be required; general consent for medical care should be considered sufficient to encompass consent for HIV testing. Repeat screening in the third trimester is recommended in certain jurisdictions with elevated rates of HIV infection among pregnant women (CDC, 2019, October 21).
Types of HIV Tests
Currently there are three types of HIV tests available: (1) nucleic acid tests (NAT), (2) antigen / antibody tests, and (3) antibody tests. They are typically performed on both blood, oral fluid, or urine.
A NAT looks for the actual virus in the blood and involves drawing blood from a vein. The test can either tell if a person has HIV or tell how much virus is present in the blood. While a NAT can detect HIV sooner than other types of tests, this test is very expensive and not routinely used for screening individuals unless they recently had a high-risk exposure or a possible exposure and have early symptoms of HIV infection (CDC, 2021, May 13).
An antigen/antibody test looks for both HIV antibodies and antigens and involves drawing blood from a vein. Antigens are foreign substances that activate the immune system. Antibodies are produced by the immune system when a person has been exposed to a foreign substance such as the HIV virus.
When a person is infected with HIV, an antigen (foreign substance) called p24 is produced even before antibodies develop. An antigen/antibody test performed by a laboratory on blood from a vein can usually detect HIV infection 18 to 45 days after an exposure. Antigen/ antibody tests done with blood from a finger prick can take longer to detect HIV (18 to 90 days after an exposure) (CDC, 2021, May 13).
HIV antibody tests only look for antibodies to HIV in blood or oral fluid. These types of tests can take 23 to 90 days to detect HIV infection after an exposure. Antibody tests that use blood from a vein can detect HIV sooner after infection than tests done with blood from a finger prick or with oral fluid. Most rapid tests and the only currently approved HIV self-test are antibody tests (CDC, 2021, May 13).
HIV Self-Tests
Did You Know. . .
The coronavirus (COVID-19) pandemic has made it more difficult for some people to access traditional places where HIV testing is provided. Self-testing allows people to get tested for HIV while still following stay-at-home orders and social distancing practices.
There are two kinds of HIV self-tests. A Rapid Self-Test is done entirely at home or in a private location and can produce results within 20 minutes. The only rapid self-test currently available in the U.S. is an oral fluid test (OraQuick) (CDC, 2021 July 16).
Mail-In Self-Tests can be ordered through various online merchant sites or through a healthcare provider. The test includes a specimen collection kit that contains supplies to collect dried blood from a fingerstick at home. The sample is then sent to a lab for testing and the results are provided by a healthcare provider (CDC, 2021, July 16).
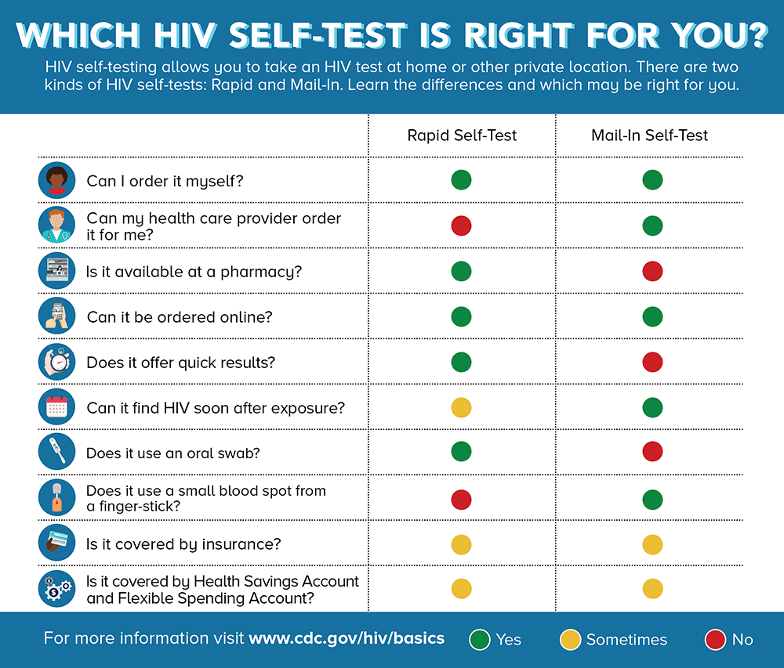
Source: CDC.
Counseling
In most states, HIV test counseling is offered to clients who are at risk for HIV or who request counseling. The goal is to help individuals assess risk, understand test results, and develop a personalized prevention plan. Counseling is an opportunity to provide education about risky behaviors, exposures, and risk-reduction strategies.
Florida law no longer requires pre-test counseling—except in cases related to pregnancy. However, the Florida Department of Health recommends that HIV testing be preceded by a pre-test counseling session that includes test purpose and procedures, information about infection and transmission, ramifications of a positive test, reducing risky behavior, available support services, and information on how to obtain test results. Florida law allows minors under the age of 18 to waive parental consent and forbids informing parents of the minor’s HIV test, results, or treatment (FDOH, 2021, July 16).
Florida law also no longer requires face-to-face post-test counseling except when test results are provided. For positive results, information must be provided on preventing transmission, availability of medical and support services, and the importance of notifying sex and/or needle-sharing partners who may have been exposed. Providers must make a good faith effort to ensure that spouses and former spouses (from the past 10 years) of HIV-infected people are notified.
For negative results, information should be provided on the prevention of HIV transmission.
The Florida Department of Health still recommends face-to-face post-test counseling that includes information about the meaning of the test results, potential consequences of a positive result, the need for retesting and risk reevaluation, support services, virus transmission, tuberculosis, and appropriate referrals.
