Although dementia has probably been around since humans first appeared on earth, it is only as we live longer that we have begun to see its widespread occurrence in older adults. The most common type of dementia is Alzheimer’s disease, but there are other types and causes of dementia. In fact, new research is suggesting that “pure” pathologies may be rare and most people likely have a mix of more than one type of dementia.
Worldwide more than 50 million people live with dementia and because people are living longer this number is expected to triple by 2050 (ADI, 2019). In Florida, there are 580,000 residents over the age of 65 currently living with Alzheimer’s disease (Alzheimer’s Association, 2023a) and by 2025, this number is expected to increase by more than 200,000.
In Florida, about 43% of residents in certified nursing homes have dementia and an additional 30% have some other psychological diagnosis (Harrington and Carrillo, 2018). This means understanding the issues and complexities associated with Alzheimer’s disease and other types of dementia is critical for family, friends, and anyone working in a nursing home, adult day care facility, or hospice, no matter what their education, training, or background.
During the COVID-19 pandemic, the number of residents living in nursing facilities in the U.S. decreased by 13%. This data reflects longer-term trends as people increasingly opt to receive care in home and community-based settings over institutional settings. COVID-19 exacerbated that trend—in part because nursing facility residents and staff incurred so many deaths during the pandemic (Chidambaram, 2022).
Defining Dementia
The ugly reality is that dementia often manifests as a relentless and cruel assault on personhood, comfort, and dignity. It siphons away control over thoughts and actions, control that we take for granted every waking second of every day.
Michael J. Passmore, Geriatric Psychiatrist,
University of British Columbia
Dementia is a collective name for the progressive, global deterioration of the brain’s executive functions. Dementia occurs primarily in later adulthood and is a major cause of disability in older adults. Although almost everyone with dementia is elderly, dementia is not considered a normal part of aging.
The exact cause of dementia is still unknown. In Alzheimer’s disease, and likely in other forms of dementia, damage within the brain is thought to be due to the formation of beta-amyloid plaques, the formation of neurofibrillary tangles, and degeneration neurons in the cerebrum. These processes are clearly explained in the following video.
Inside the Brain: Unraveling the Mystery of Alzheimer’s Disease [4:22]
National Institute on Aging. https://www.youtube.com/watch?v=NjgBnx1jVIU
In Alzheimer’s disease, damage begins in the temporal lobe, in and around the hippocampus. The hippocampus is part of the brain’s limbic system and is responsible for the formation of new memories, spatial memories and navigation, and is also involved with emotions.
Mild Alzheimer’s Disease
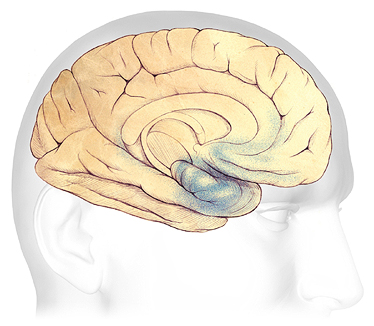
In the earliest stages of Alzheimer’s disease, before symptoms can be detected, plaques and tangles form in and around the hippocampus, part of the brain’s limbic system (shaded in blue).
Source: Courtesy of The Alzheimer’s Association. Used with permission.
As the disease progresses, plaques and tangles spread to the front part of the brain (the temporal and frontal lobes). These areas of the brain are involved with language, judgment, and learning. Speaking and understanding speech, the sense of where your body is in space, and executive functions such as planning and ethical thinking are affected.
Moderate Alzheimer’s Disease
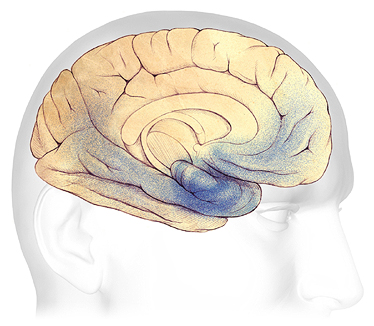
In mild to moderate stages, plaques and tangles (shaded in blue) spread from the hippocampus forward to the frontal lobes.
Source: Courtesy of The Alzheimer’s Association. Used with permission.
In severe Alzheimer’s disease, damage is spread throughout the brain. Notice in the illustration below the damage (dark blue) in the area of the hippocampus, where new, short-term memories are formed. At this stage, because so many areas of the brain are affected, individuals lose their ability to communicate, to recognize family and loved ones, and to care for themselves.
Severe Alzheimer’s Disease
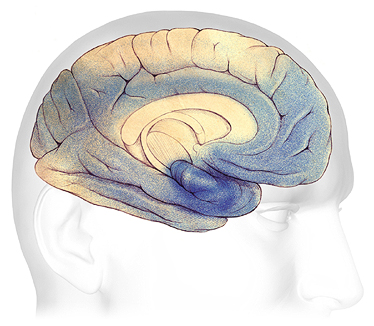
In advanced Alzheimer’s, plaques and tangles (shaded in blue) have spread throughout the cerebral cortex.
Source: Courtesy of The Alzheimer’s Association. Used with permission.
Changes in the brain may begin a decade or more before symptoms appear. During the very early stage of Alzheimer’s, toxic changes are beginning to affect the brain, including abnormal buildups of proteins that form amyloid plaques and tau tangles. Previously healthy neurons damaged by these abnormalities lose connections with other neurons and eventually die. As these changes progress, cognitive changes begin to occur.
Types of Dementia
Although Alzheimer’s disease is the most common cause of dementia, it isn’t the only cause. Frontotemporal degeneration—which begins in the frontal lobes—is a relatively common type of dementia in those under the age of 60. Vascular dementia and Lewy body dementia are other common types of dementia (see table). In all, more than twenty different types of dementia have been identified.
Some Common Types of Dementia | |||
|---|---|---|---|
Dementia subtype | Characteristic symptoms | Neuropathology | % of cases |
*Alzheimer’s disease (AD) |
|
| 60%–80% |
Frontal-temporal dementia |
|
| 5%–10%, prevalence thought to be underestimated |
*Vascular dementia |
|
| 20%–30% |
Dementia with Lewy bodies (closely related to Parkinson’s disease dementia) |
|
| ~5%-10% |
Diagnostic Guidelines
Diagnosis of Alzheimer’s disease and other types of dementia is based primarily on clinical signs and symptoms; there is no single test or technique that can diagnose dementia. A combination of tools such as medical history, neurological exams, cognitive and functional assessments, brain imaging (MRI, CT, PET) and cerebrospinal fluid or blood tests are increasingly being used to make an accurate diagnosis (Alzheimer’s Association, 2023b).
To guide clinicians, in 2011 the National Institute on Aging and the Alzheimer’s Association (NIA-AA) published updated earlier diagnostic guidelines to provide a deeper understanding Alzheimer’s disease. The 2011 guidelines:
- Recognize that Alzheimer’s disease progresses on a spectrum with three stages: (1) an early, preclinical stage with no symptoms; (2) a middle stage of mild cognitive impairment; and (3) a final stage marked by symptoms of dementia. Cognitive decline is gradual and progressive.
- Expand the criteria for Alzheimer’s dementia beyond memory loss as the first or only major symptom and recognize that other aspects of cognition, such as word-finding ability or judgment, may become impaired first. Other findings can include changes in:
- episodic memory
- executive functioning
- visuospatial abilities
- language functions
- personality and/or behavior
- Reflect a better understanding of the distinctions and associations between Alzheimer’s and non-Alzheimer’s dementias, as well as between Alzheimer’s and disorders that may influence its development, such as vascular disease, delirium, or stroke.
- Recognize the potential use of biomarkers—indicators of underlying brain disease—to diagnose Alzheimer’s disease. However, the guidelines state that biomarkers are almost exclusively to be used in research rather than in a clinical setting.
National Institute on Aging, 2020
Since the publication of the 2011 guidelines, researchers have increasingly come to understand that cognitive decline in AD occurs continuously over a long period, and that progression of biomarker measures* is also a continuous process that begins before symptoms are evident. The disease is now regarded as a continuum rather than three distinct clinically defined stages (Jack et al., 2018).
*β amyloid deposition, pathologic tau, and neurodegeneration/neuronal injury.
A 2018 update of the 2011 NIA-AA diagnostic guidelines added a “numerical clinical staging scheme.” This staging scheme reflects the sequential evolution of AD from an initial stage characterized by the appearance of abnormal biomarkers in asymptomatic individuals. As biomarker abnormalities progress, the earliest subtle symptoms become detectable. Further progression of biomarker abnormalities is accompanied by progressive worsening of cognitive symptoms, culminating in dementia (Jack et al., 2018).
The numerical clinical staging scheme is as follows:
- Performance within expected range on objective cognitive tests.
- Normal performance within expected range on objective cognitive tests. (Transitional cognitive decline: Decline in previous level of cognitive function, which may involve any cognitive domains.
- Performance in the impaired/abnormal range on objective cognitive tests.
- Mild dementia.
- Moderate dementia.
- Severe dementia.
Jack et al., 2018
An Alzheimer’s Association workgroup published a report describing the need for clinical practice guidelines for use in primary and specialty care settings. The guidelines build on the NIA_AA guidelines but add a clinical component for the evaluation of cognitive impairment thought to be related to Alzheimer’s disease or a related type of dementia.
Key components include:
- All middle-aged or older individuals who self-report or whose care partner or clinician report cognitive, behavioral, or functional changes should undergo a timely evaluation.
- Concerns should not be dismissed as “normal aging” without a proper assessment.
- Evaluation should involve not only the patient and clinician but, almost always, also involve a care partner (e.g., family member or confidant).
Atri, 2019
Brain Imaging
Brain imaging such as structural imaging, functional imaging, and molecular imaging, are increasingly being used to assist with the early diagnosis of Alzheimer’s disease and related dementias by detecting visible, abnormal structural, functional, or cellular changes in the brain.
Structural imaging uses magnetic resonance imaging (MRI) and computed tomography (CT) to provide information about the shape, position, and volume of the brain. It can be used to detect brain shrinkage, which is related to excessive nerve death. Structural imaging can also be used to reveal tumors, evidence of small or large strokes, damage from severe head trauma, or a buildup of fluid in the brain, as well as detect underlying conditions that may preclude an individual from certain treatments (Alzheimer’s Association, 2023b).
Molecular Imaging Using a PET Scan
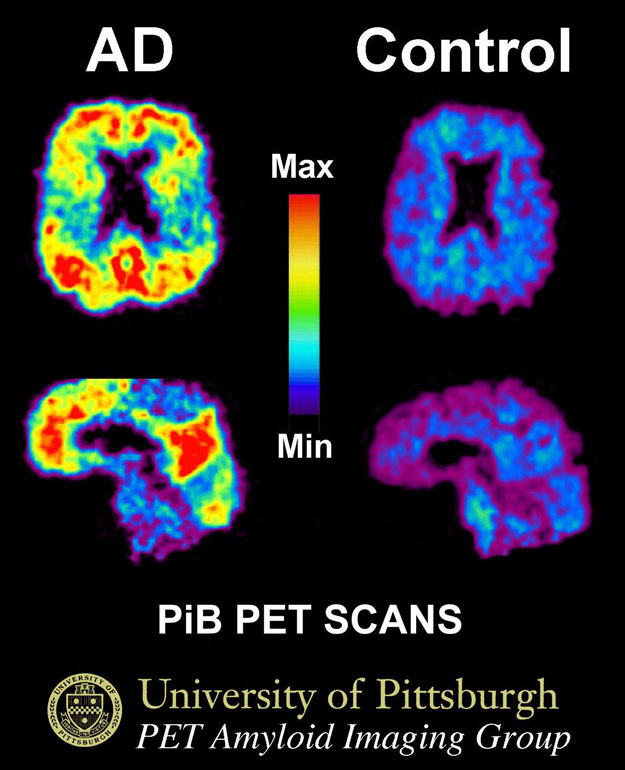
This PET scan shows show the uptake of a radiotracer called Pittsburg compound B (PiB). A patient with Alzheimer's disease is shown on the left and an elderly person with normal memory on the right. Areas of red and yellow show high concentrations of PiB in the brain and suggest high amounts of amyloid deposits in these areas. Source: By Klunkwe—Own work, CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=5470244.
Functional imaging uses positron emission tomography (PET) and functional MRI (fMRI) to understand how cells in various brain regions are working by showing how actively the cells use sugar or oxygen. Studies has indicated that Alzheimer's is often associated with reduced use of glucose in areas of the brain associated with memory, learning, and problem-solving (Alzheimer’s Association, 2023b).
Molecular imaging uses PET and fMRI and targeted radiotracers to detect cellular or chemical changes linked to specific diseases. Four molecular imaging compounds have been approved for clinical use: Florbetaben, Florbetapir, and Flutemetamol have been approved for detection of beta-amyloid in the brain. Flortaucipir F18 has been approved for detection of tau in the brain (Alzheimer’s Association, 2023b).
Biomarkers
Recent research has focused on discovering, evaluating, and validating biomarkers for application in clinical research. The goal is to provide evidence for earlier diagnostic and prognostic capability. Biomarkers are also employed to confirm and improve on diagnostic accuracy of dementia. In Alzheimer’s disease, biomarker development and validation has focused primarily on cerebrospinal fluid (CSF), and PET to detect central nervous system amyloid beta (Aβ) or tau/tangles—the two pathological hallmarks of AD (Turner et al., 2020).
CSF biomarkers are measures of the concentrations of proteins in cerebral spinal fluid from the lumbar sac. They reflect the rates of both production (protein expression or release/secretion from neurons or other brain cells) and clearance (degradation or removal) at a given point in time.
Best practices for the use of CSF in patients with suspected dementia include: (1) determine whether CSF testing for AD biomarkers is appropriate for the patient; (2) educate the patient and family about the benefits and risks of testing, and assess their motivation and psychological readiness to learn more about their risk of AD; (3) ensure the procedure is performed with reliable assays following established guidelines; and (4) integrate the results into the evaluation and treatment plan in an in-person meeting with the patient and family (Shaw et al., 2018).
Sensory Impairment and the Changing Brain
Sensory impairments are often overlooked by caregivers and healthcare professionals when interacting with an older adult with dementia. Hearing, visual, and somatosensory impairments must be considered when assessing difficult behaviors, as well as a person’s ability to complete common activities of daily living.
For a person with dementia, impairments related to hearing and vision are associated with adverse outcomes. For example, hearing impairment is associated with poor self-rated health, difficulties with basic and instrumental activities of daily living, difficulty with memory, frailty, and falls (Guthrie et al., 2018).
Similarly, visual impairment has been linked to an increased risk of mortality, difficulties with activities of daily living and mobility, and reduced social participation. Individuals with visual impairment also are more likely than those without visual impairment to require community-based supports (Guthrie et al., 2018).
Loss of cells in the part of the brain that processes vision (occipital lobe) causes a narrowing of the visual field and a loss of peripheral vision. Vision narrows and becomes binocular, and items placed on a table in front of a person (such as food) may be outside a person’s visual field. Vision (especially peripheral vision) is critical for good balance and visual impairment means a person must rely heavily on touch to help with balance.

Because of damage to the visual pathways in the brain, the visual field narrows, making it difficult to see above, beside, and below. Source: National Eye Institute, National Institutes of Health. Public domain.
Macular degeneration, a common visual impairment in older adults causes loss of vision in the center of the eye, making it difficult to see something directly in front of you.

Normal vision on the left and damage cause by macular degeneration on the right. Source: National Eye Institute, National Institutes of Health. Public domain.
Conditions That Can Mimic Dementia
Medical conditions other than dementia can cause cognitive changes in older adults. Gerontology specialists speak of the “3Ds”—dementia, delirium, and depression—because these three conditions are common reasons for cognitive changes in older adults. Delirium and depression are often mistaken for dementia.
Delirium
Delirium is a sudden, severe confusion with rapid changes in brain function. Along with dementia, it is the most common cause of altered mental status in older adults (Gogia and Fang, 2023). Delirium has an abrupt onset, developing over hours or days and is typically temporary and reversible. It mainly affects attention but also is associated with changes in perception, mood, and cognition.
The most common causes of delirium in people with dementia are medication side effects, low or high blood sugar, fecal impactions, urinary retention, electrolyte disorders and dehydration, infection, stress, and metabolic changes. An unfamiliar environment, injury, or severe pain can also cause delirium. As a care provider with direct, daily contact with clients, your observations and feedback help other healthcare providers identify changes that may be treatable.
Delirium and dementia often co-exist although the pathophysiology remains poorly understood (Gogia and Fang, 2023). Delirium is under-diagnosed in almost two-thirds of cases and can be misdiagnosed as depression or dementia. Since the most common causes of delirium are reversible, recognition enhances early intervention. Early diagnosis can lead to rapid improvement (Hope et al., 2014).
Depression
Depression is a common but serious mood disorder. Major depressive disorder is characterized by a combination of symptoms that interfere with a person's ability to work, sleep, study, eat, and enjoy activities. Some people may experience only a single episode within their lifetime, but more often a person may have multiple episodes (NIMH, 2023).
Persistent depressive disorder (also called dysthymia or dysthymic disorder) is characterized by long-term (2 years or longer) symptoms that may not be severe enough to disable a person but can prevent normal functioning or feeling well. Depression with symptoms of psychosis occurs when a person has severe depression, plus some form of psychosis—such as delusions or hallucinations. Psychotic symptoms typically have a depressive “theme,” such as delusions of guilt, poverty, or illness (NIMH, 2023).
Depression is very common in people with dementia. Depressive symptoms can cause distress, decrease quality of life, and exacerbate cognitive and functional impairment. It is often undiagnosed and untreated in people with dementia living in nursing homes, even though a high prevalence of depression has been reported for nursing home residents (O’Sullivan et al., 2022).
Comorbid depression in a person with dementia is associated with a profound decrease in quality of life, accelerated cognitive decline, increased mortality, and caregiver stress. The diagnosis and treatment of depression in people with dementia should be a clinical priority (O’Sullivan et al., 2022).
Even in the absence of dementia, depression can cause a person to feel hopeless, worthless, or helpless. Feelings of sadness, anxiety, or emptiness are common in addition to loss of interest in activities that were once pleasurable. Decision-making, concentration, and memory can also be affected. Because these symptoms often overlap with cognitive decline, caregivers may fail to recognize the difference between symptoms of depression and dementia and therefore offer no treatment. Other symptoms of depression can include:
- Irritability, restlessness, or having trouble sitting still.
- Moving or talking more slowly.
- Thoughts of death or suicide, or suicide attempts.
- Aches or pains, headaches, cramps, or digestive problems without a clear physical cause and/or that do not ease with treatment.
NIA, 2021
