Nurse Jennifer was hesitant to complete the incident report because she didn’t want to be in trouble with her charge nurse; besides, she didn’t even have time to complete the report. Besides, she thought, the patient with DKA didn’t have any bloodborne pathogens. The DKA was caused by the poor healing leg wounds. Her only personal concern was for the patient with COPD, who had known hepatitis C.
In reviewing the history of the DKA patient, Jennifer read that he had tested HIV-positive, which had complicated the healing of his right leg wounds and severely elevated his blood sugar levels. Suddenly the innocent needle stick looked scary. Panicked and afraid, she went to her charge nurse to disclose what had happened.
What is the protocol for an accidental needle stick? Whose responsibility is it to address the problem?
The federal OSHA Bloodborne Pathogens Standard specifies that “each employer having an employee(s) with occupational exposure shall establish a written Exposure Control Plan designed to eliminate or minimize employee exposure.” Paragraph (b) says:
Occupational exposure means reasonably anticipated skin, eye, mucous membrane, or parenteral contact with blood or other potentially infectious materials that may result from the performance of an employee's duties. (OSHA, 2019a)
In other words, if any workers may reasonably expect to have contact with blood or body fluids on the job, this law applies to their workplace. All healthcare workers—both licensed and non-licensed—risk exposure to bloodborne pathogens, whether they work in hospitals, nursing homes, home care, or correctional institutions.
Work Areas of Special Concern
Research has identified home care and correctional institutions as work areas with increased risk of bloodborne pathogen transmission. Work practices such as extended work schedules and understaffing also increase the risk of percutaneous injuries (Phillips, et al., 2012). The good news is that after OSHA enacted the needle stick safety and prevention training known as the Needle stick Safety and Prevention Act of 2000, the incidence of worker injuries from needle sticks as declined, however it is also estimated that 50% of all sharps injuries go unreported.
Home Care
Earlier discharge from hospitals means that patients are going home “sicker and quicker,” and may have health needs that demand complex nursing skills. Studies show that both RNs and aides/personal care assistants (PCAs) are still experiencing sharps injuries at significant levels. One study found that 14% of RNs reported one or more sharps injuries in the previous three years. These injuries were associated with lack of compliance with Standard Precautions, recapping of needles, exposure to household stressors, exposure to violence, and mandatory overtime (CDC, 2013).
Another study found that PCAs are at increased risk when performing nursing-related activities for which they are inexperienced and/or lack training (Lipscomb et al., 2009). A third study showed that 35% of nurses and 6.4% of aides experienced at least one sharps injury during their home healthcare career. It is estimated approximately 800,000 needle sticks occur each year by healthcare professionals (CCOH, 2018).
Procedures contributing to sharps injuries obviously include injecting medications with needles, administering finger sticks and heel sticks, and drawing blood. Sharps disposal, contact with waste, and patient handling also contributed to sharps injuries. Newer devices are available and being developed to help healthcare staff avoid needle sticks; they include needle-shielding devices, auto-disabling systems to prevent the reuse of needles, and retraction of needles by pushing a clicker button built within the syringe.
Yet another study evaluated the experiences of 355 home healthcare nurses and 30 Medicare Certified Home Healthcare Agency and hospice employers in one state and found that some employer policies and nurse practices were out of compliance with OSHA, and they were experiencing needle sticks from nonadherence to established safety standards.
There is also a discrepancy between needle sticks being reported and those actually occurring. Thirty-eight home healthcare nurses from 12 of the 30 employers reported sharps injuries within the past year but the employers reported only 18 such injuries in that same year (Scharf et al., 2009). More effective education, training, and enforcement of OSHA standards are needed to reduce the incidence of sharps injuries in these areas of practice.
An Exposure Control Plan that includes safety sharps and training on how to use them correctly is required by the Bloodborne Pathogens Standard (OSHA, 2019c). Talk with your employer if you are concerned about exposure risk on the job or would like the newer products being developed to become available in your facility.
Sharp Object Injuries by Medical Personnel
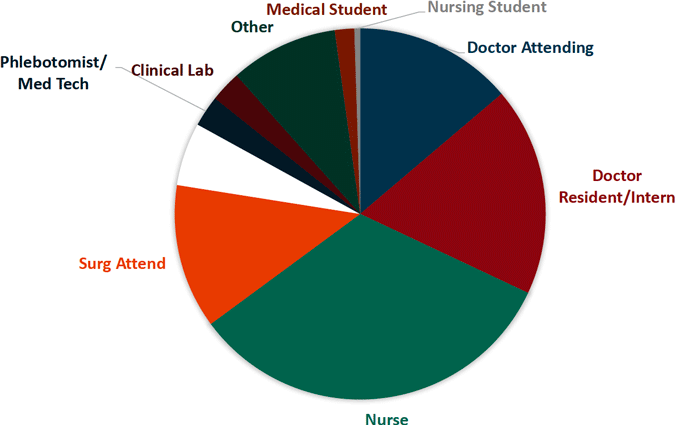
Sharp Object Injuries by Type of Device
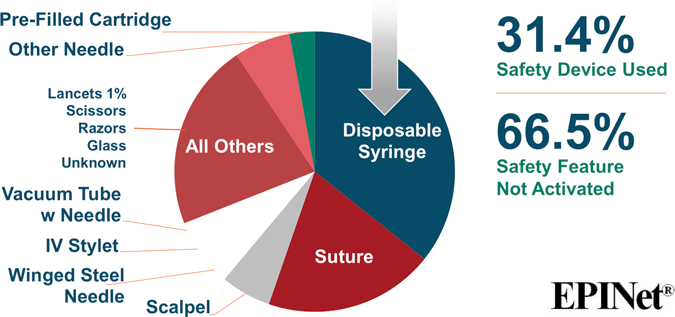
Source for both images: EPINet, 2017.
A Message About Sharps Safety: Protecting Against Needle Sticks and Other Sharps Injuries [3:42]
https://www.youtube.com/watch?v=nv_2S0p9Wj0
Correctional Institutions
All healthcare workers risk occupational exposure to bloodborne pathogens but those who work in correctional facilities face additional challenges:
- Jails and prisons are unpredictable work settings.
- Security issues are often a higher priority than infection control.
- Inmates may have a higher rate of bloodborne diseases. The rate of AIDS in prison is 5 to 7 times the rate in the U.S. general population.
- Almost 25% of all those living with HIV are in a correctional facility. (AIDS Infonet, 2014)
Correctional healthcare workers may be bitten or stabbed during an inmate assault, punctured with a used needle, or splashed in the face with blood. Any of these situations can expose workers to bloodborne diseases (Hood, 2011). Despite security measures, illicit drugs still enter correctional facilities for inmate recreational drug use. It is estimated that a single needle will be re-used 200 times among 100 inmates, causing a serious infectious risk for anyone with a needle stick. Education and training of correctional healthcare workers is essential to prevent exposure in these high-risk work settings.
In the past decade, the U.S. Food and Drug Administration (FDA) approved a needle-free injection system that pushes medicine directly through the skin without a needle. One product, among many being developed, has been used in correctional facilities and for mainstream medications such as insulin, epinephrine and even flu vaccines, which demonstrates a hopeful solution (Hood, 2011). According to the OSHA Bloodborne Pathogen Standard, the use of safer medical devices must be implemented, and employees must be trained for their proper use (OSHA, 2019d). OSHA does not dictate which products a facility must use, but the standard does state that if safety devices and better products are available that promote safety, the facility has the obligation to evaluate and use them.
Research and Production Facilities
The OSHA Bloodborne Pathogens Standard includes a section on protection of workers in specialized types of worksites such as research and production facilities and pharmaceutical facilities where risk of exposure to HBV and HIV is significantly higher. Protective measures for these worksites are much more stringent. If your workplace is not this type of facility, these more stringent requirements do not apply to it (OSHA, 2019d).
Exposure Control Plans
Employers are required to create and implement a written exposure control plan (ECP) specific to each workplace to eliminate or minimize employee exposures. The plan must be updated annually to reflect technological changes (eg, newer needless syringes) that help eliminate or reduce exposure to bloodborne pathogens. In the plan, employers must include information about the ECP used in the workplace.
The ECP should contain annual documentation of consideration and implementation of commercially available safer medical devices designed to eliminate or minimize occupational exposure. Employers must also document that they have solicited input from non-managerial workers in identifying, evaluating, and selecting engineering controls. The ECP must be available to workers. You have the legal right to ask your employer how you can review it.
The ECP for your facility must:
- Be written specifically for each facility
- Be reviewed and updated yearly
- Be readily available to all workers
- Include regular education for workers
The exposure control plan should also include a written exposure process that includes those job classifications and positions in which employees have the potential for occupational exposures. The exposure determination should be made without consideration of the use of PPE or equipment. Employees who are required or expected to administer first aid must also be included.
In addition to the possible presence in blood, bloodborne pathogens may be present in other potentially infectious material (OPIM). OPIM includes:
- Human body fluids: semen, vaginal secretions, cerebrospinal fluid, synovial fluid, pleural fluid, pericardial fluid, peritoneal fluid, amniotic fluid, saliva in dental procedures, any body fluid that is visibly contaminated with blood, and all body fluids in situations where it is difficult or impossible to differentiate between body fluids
- Any unfixed tissue or organ (other than intact skin) from a human (living or dead)
- HIV-containing cell or tissue cultures, organ cultures, and HIV- or HBV-containing culture medium or other solutions; and blood, organs, or other tissues from experimental animals infected with HIV or HBV
OSHA’s Bloodborne Pathogens Law requires Universal Precautions for the OPIM listed above. Note that the list does not specify precautions with urine or feces, which may be heavily loaded with bacteria other than the bloodborne pathogens. Universal Precautions should absolutely be implemented for all body fluids and waste products and considered potentially infectious.
Standard Precautions, as described by the CDC, are broader than Universal Precautions, covering more bacteria and viruses than the three main bloodborne pathogens—HBV, HCV, and HIV. Standard Precautions specify that contact with all body fluids from all patients should be avoided and considered potentially infectious. Often these names are used synonymously in clinical practice although they are technically different, however both focus on protection by the healthcare worker from all body fluids from another person.
OSHA requires the use of Engineering Controls, Work Practice Controls, and Personal Protective Equipment—in that order, because the most effective protections for workers take priority.
Test Your Knowledge
Research on bloodborne pathogens has identified the following areas of special concern:
- Daycare and playgrounds.
- Classrooms and sports facilities.
- Obstetrical units.
- Correctional facilities and research facilities.
Employers are required to:
- Create a written exposure control plan that must be updated every 2 years to minimize employee bloodborne pathogen exposures.
- Make an exposure report that evaluates the effectiveness of personal protective equipment used by nurses.
- Consider and implement appropriate, commercially available safer medical devices to eliminate or minimize occupational exposure.
- Make an exposure determination on all patients who may be considered at risk for a bloodborne pathogen exposure.
Apply Your Knowledge
Have you seen a copy of your facility’s ECP? Is your facility educating you about bloodborne pathogen prevention? Is your facility providing a needless entry system for medications? Who is in charge of evaluating new products for your facility? Have you seen some of the new needless injection products?
Answers: D, C
