Error is defined as the failure of a planned action to be completed as intended or the use of a wrong plan to achieve an aim.
Institute of Medicine [sic], 1999
To Err Is Human: Building a Safer Health System
There are many ways that medical care can go wrong. Errors can occur around the administration of medications, during laboratory testing, when infections occur within the healthcare setting, as a result of surgery, in an environment that contributes to pressure sores or a patient fall, or even in documentation or data entry tasks.
A number of healthcare organizations and government agencies have lists of medical errors on which they focus, but the seven discussed here appear across lists from most oversight organizations and are the ones most commonly encountered:
- Medication events (including adverse drug events/reactions)
- Healthcare-associated infections (HAIs)
- Surgical errors
- Laboratory errors
- Patient Falls
- Pressure sores
- Documentation/computer errors (NQF, 2011; AHRQ, 2018; CMS, 2018; Joint Commission, 2016; NHSN, 2019)
Medical errors are everyone’s business and everyone’s responsibility. Whether you are a healthcare professional, a family caregiver, or a patient, the more you know, the better you can protect yourself and others. How much you need to know varies with your situation.
Nursing professionals need a wider range of information about medication errors, for example, but every occupational or physical therapist will be better able to observe and protect their patients if they possess an appropriate understanding of the effects and symptoms of medication problems. The same is essentially true across all categories of medical errors.
An individual may not need to know as much detail as the healthcare professional, but the bottom line is that we each must be advocates for our own healthcare. We need to be prepared to recognize potential problems and ask questions of our healthcare providers, and to know when to act.
Our culture has not always been one that promotes questioning of authority figures but, as with all things human, errors can happen in healthcare and those errors can have life changing or life ending consequences. In late November 2018, a lengthy investigation by the Tampa Bay Times into a pattern of deadly errors at All Children’s Heart Institute in Tampa, Florida, brought to light the many ways in which error can creep in and be compounded and we may not be prepared to question and report soon enough to prevent tragedy (McGrory & Bedi, 2018).
Medication Events
Eighty-two percent of American adults take at least one medication and 29% take five or more—and the potential for medication events is likely to grow.
Medication Errors
The National Coordinating Council for Medication Error Reporting and Prevention (NCCMERP) defines a medication error as:
any preventable event that may cause or lead to inappropriate medication use or patient harm while the medication is in the control of the healthcare professional, patient, or consumer. Such events may be related to professional practice, healthcare products, procedures, and systems, including prescribing; order communication; product labeling, packaging, and nomenclature; compounding; dispensing; distribution; administration; education; monitoring; and use (NCCMERP, 2019; USFDA, 2018).
Medication errors can occur throughout the medication-use system, such as when prescribing a drug; upon entering information into a computer system; when the drug is being prepared or dispensed; or when the drug is given to or taken by a patient. The U.S. Food and Drug Administration (FDA) receives more than 100,000 U.S. reports each year associated with a suspected medication error (USFDA 2018).
Common causes of such errors include:
- Poor communication,
- Ambiguities in product names, directions for use, medical abbreviations or writing,
- Poor procedures or techniques, or
- Patient misuse because of poor understanding of the directions for use of the product.
In addition, job stress, lack of product knowledge or training, or similar labeling or packaging of a product may be the cause of, or contribute to, an actual or potential error (USFDA, 2017).
Not all medication errors result in harm to the patient. For example, if the dosage or route were prescribed incorrectly but the error was caught prior to administration (often called a “near miss”), there was no patient harm. That said, any type of medication error must be tracked so preventions can be developed, regardless of whether a patient was harmed.
Adverse Drug Events (ADEs)
Adverse drug events are harms resulting from the use of medication and include allergic reactions, side effects, overmedication, and medication errors (CDC, 2018).
Adverse drug events (ADEs) cause an estimated 1.3 million emergency department visits each year and $3.5 billion is spent on excess medical costs of ADEs annually. The CDC notes that the numbers of ADEs will likely grow, due to development of new medications, discovery of new uses for older medications, an aging American population, increase in the use of medications for disease prevention, and increased coverage for prescription medications (CDC, 2018a).
According to the federal Office of Disease Prevention and Health Promotion, ADEs are responsible for a staggering number of harmful patient impacts.
In inpatient settings, ADEs:
- Account for an estimated 1 in 3 of all hospital adverse events
- Affect about 2 million hospital stays each year
- Prolong hospital stays by 1.7 to 4.6 days
In outpatient settings, ADEs account annually for:
- Over 3.5 million physician office visits
- An estimated 1 million emergency department visits
- Approximately 125,000 hospital admissions (ODPHP, 2019)
In addition, the CDC notes that ADEs are responsible for $3.5 billion in extra medical expenses as well as 40% of preventable ambulatory care costs (CDC, 2018).
A large majority of ADEs are preventable. It is believed that reducing ADEs will have numerous benefits including safer and higher quality healthcare services, reduced healthcare costs, more informed and engaged consumers, and improved health outcomes (ODPHP, 2019).
Reporting an Adverse Drug Event
MedWatch, the FDA's safety information and adverse event reporting program, plays a critical role in the agency's post marketing surveillance—the process of following the safety profile of medical products after they've begun to be used by consumers. Through MedWatch, a voluntary program, health professionals report adverse reactions, product problems, and use errors related to drugs, biologics, medical devices, dietary supplements, cosmetics, and infant formulas (USFDA, 2016).
The MedWatch program has two main goals:
- Facilitate the reporting of problems
- Get safety information out to the public (USFDA, 2016)
There are three ways to report an adverse event to MedWatch:
- Complete the voluntary Form FDA 3500 online here.
- Call 800 FDA 1088 to report by telephone.
- Download a reporting form: Form FDA 3500 or 3500B (consumer/patient form) and either fax it to 800 FDA 0178 or mail it (USFDA, 2018b).
Adverse Drug Reactions (ADRs)
While all adverse drug reactions are adverse drug events, not all adverse drug events are adverse drug reactions. A patient given the wrong medication is an adverse drug event but not an adverse drug reaction because the medication in question was not used as it was intended. The figure below helps to clarify how the medication-related errors differ and relate to one another.
Adverse Drug Reactions (ADRs)
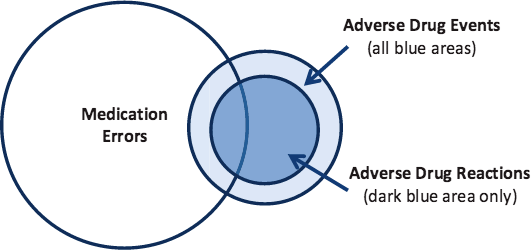
Source: ODPHP, 2014.
Adverse drug reactions are a leading cause of injury and death; it is estimated they cause 100,000 deaths annually in the United States (USFDA, 2018).
More than 2 million serious ADRs occur each year, 350,000 in nursing homes alone. In recent years the number of medications prescribed to patients has increased dramatically and, not surprisingly, adverse drug reactions have also increased (USFDA, 2018).
There are three main causes for adverse reactions:
- As many as two-thirds of all patient visits to a doctor result in a prescription, and there are more drugs and combinations of drugs being used than ever before.
- More than 4 billion prescriptions were filled in 2017 at retail pharmacies alone, nearly 10 prescriptions per person in the United States.
- ADRs increase exponentially when a patient is taking four or more medications (USFDA, 2018; KFF, 2018).
The drug approval process may also play a role in the increase of adverse drug reactions. A drug that is tested in only a few thousand people may have an excellent safety profile in those patients, but some drugs require many more exposures to detect an adverse reaction—particularly reactions that occur with low frequencies.
According to the FDA learning module Preventable Adverse Drug Reactions: A Focus on Drug Interactions, most drugs are approved for use by the FDA with an average of only 1,500 patient exposures and tested for a relatively short period of time. A few million patients may take a new drug before the low-frequency adverse reactions are identified. For drugs that cause rare toxicity, the toxicity will only be detected after use by many more thousands of patients (USFDA, 2018a).
Reducing Adverse Drug Reactions Through Technology
Adverse drug reactions can be detected and prevented through systems intervention. Tools such as computerized physician orders and prescription entry (CPOE) and barcoding systems have taken the guess work out of reading written prescriptions for nurses and pharmacists. Medication errors can be reduced potentially through the use of electronic health records (EHRs) as well as drug-interaction screening software that detects and alerts the physician and pharmacist to potentially serious drug interactions.
The HITECH Act’s meaningful use policy has specific medication management measures:
- Using computerized provider order entry (CPOE) systems for medication orders
- Implementing decision support systems to check for drug–drug and drug–allergy interactions
- Having the capability to electronically exchange key clinical information (such as medication lists, medication allergies, and test results) with other providers
- Maintaining an active medication list, and
- Maintaining an active medication allergy list (Murphy & White, 2014)
Closer to home, a study funded by AHRQ found Florida hospitals that adopted all five core measures of meaningful use for medication management in 2010 had the lowest rate of adverse drug events of all hospitals in the state. Intriguingly, hospitals where physicians objected to adopting HITECH’s meaningful use measures for medication management saw their adverse drug events increase by 14%, compared to a 52% reduction at hospitals where physicians supported the medication management meaningful use measures (Murphy & White, 2014).
The AVOID Mnemonic
Clinicians cannot rely solely on technology to prevent errors in prescribing and administering of medications. Frequent consultation with other members of the healthcare team is invaluable.
Use of an organized, step-wise approach also helps prevent drug interactions. The AVOID mistakes mnemonic can be used to collect all necessary information for the medication history (see table below).
AVOID Mnemonic | |
|---|---|
Keyword | What to Ask |
Allergies | Ask the patient if there is any drug that should not be prescribed for any reason. |
Vitamins or herbs | Ask the patient whether the patient is taking or has a reaction to any herb, vitamin, or “alternative” or “natural” product. |
Old drugs and over the counter (OTC) drugs…in addition to all current drugs | Ask about old drugs (prescription and OTC) as well as current drugs the patient is taking. Some of these drugs may have relatively long-lasting effects (either toxicity or potential for drug interactions). |
Interactions | Evaluate the potential for adverse drug interactions. Consider a behavioral contract between the physician and the patient in an effort to help the patient reach the therapeutic goal, either in the case of drug dependence or adherence to a therapeutic regimen, with a clear plan. |
Dependence potential | Is the patient drug dependent or at risk of dependence on, for example, opioids, benzodiazepines, alcohol, or other substances of abuse. Consider a behavioral contract between the physician and the patient in an effort to help the patient reach the therapeutic goal, both in the case of drug dependence and in adherence to a therapeutic regimen. |
Mendel (genetics) | Genetics: Is there a family history of benefits from or problems with any drugs? |
Specific Preventive Measures
Naming, Labeling, Packaging, and Abbreviations
The FDA looks for ways to prevent medication errors. Before drugs are approved for marketing, it reviews the drug name, labeling, packaging, and product design to identify and revise information that may contribute to medication errors. For example, the FDA reviews:
- Proposed proprietary (brand) names to minimize confusion among drug names. Using simulated prescriptions and computerized models, it determines the acceptability of proposed proprietary names to minimize medication errors associated with product name confusion.
- Container labels to help healthcare providers and consumers select the right drug product. If a drug is made in multiple strengths the labels of each container should be easy to differentiate. The label design may use different colors or identify the strength in large bold numbers and letters.
- Prescribing and patient information to ensure the directions for prescribing, preparing, and use are clear and easy to read.
After drugs are approved for marketing in the United States, the FDA monitors and evaluates medication error reports. It may require a manufacturer to revise the labels, labeling, packaging, product design or proprietary name to prevent medication errors. The FDA may also issue communications alerting the public about a medication error safety issue with a variety of communications (FDA, 2018).
The FDA recommends that clinicians review the Institute for Safe Medical Practices’ List of Error-Prone Abbreviations, Symbols and Dose Designations as shown in the following tables.
Dangerous Abbreviations | |||
|---|---|---|---|
Abbreviation | Intended Meaning | Misinterpretation | Correction |
| µg | Microgram | Mistaken as “mg” | Use “mcg” |
| AD, AS, AU | Right ear, left ear, each ear | Mistaken as OD, OS, OU (right eye, left eye, each eye) | Use “right ear,” “left ear,” or “each ear” |
| OD, OS, OU | Right eye, left eye, each eye | Mistaken as AD, AS, AU (right ear, left ear, each ear) | Use “right eye,” “left eye,” or “each eye” |
| BT | Bedtime | Mistaken as “BID” (twice daily) | Use “bedtime” |
| cc | Cubic centimeters | Mistaken as “u” (units) | Use “mL” |
| D/C | Discharge or discontinue | Premature discontinuation of medications if D/C (intended to mean “discharge”) has been misinterpreted as “discontinued” when followed by a list of discharge medications | Use “discharge” and “discontinue” |
| IJ | Injection | Mistaken as “IV” or “intrajugular” | Use “injection” |
| IN | Intranasal | Mistaken as “IM” or “IV” | Use “intranasal” or “NAS” |
| HS | Half-strength | Mistaken as bedtime | Use “half-strength” or “bedtime” |
| hs | At bedtime, hours of sleep | Mistaken as half-strength | Use “half-strength” or “bedtime” |
| IU** | International unit | Mistaken as IV (intravenous) or 10 (ten) | Use “units” |
| o.d. or OD | Once daily | Mistaken as “right eye” (OD-oculus dexter), leading to oral liquid medications administered in the eye | Use “daily” |
| OJ | Orange juice | Mistaken as OD or OS (right or left eye); drugs meant to be diluted in orange juice may be given in the eye | Use "orange juice" |
| Per os | By mouth, orally | The “os” can be mistaken as “left eye” (OS-oculus sinister) | Use “PO,” “by mouth,” or “orally” |
| q.d. or QD** | Every day | Mistaken as q.i.d., especially if the period after the “q” or the tail of the “q” is misunderstood as an “i” | Use “daily” |
| qhs | Nightly at bedtime | Mistaken as “qhr” or every hour | Use “nightly” |
| qn | Nightly or at bedtime | Mistaken as “qh” (every hour) | Use “nightly” or “at bedtime” |
| q.o.d. or QOD** | Every other day | Mistaken as “q.d.” (daily) or “q.i.d. (four times daily) if the “o” is poorly written | Use “every other day” |
| q1d | Daily | Mistaken as q.i.d. (four times daily) | Use “daily” |
| q6PM, etc | Every evening at 6 PM | Mistaken as every 6 hours | Use “daily at 6 PM” or “6 PM daily” |
| SC, SQ, sub q | Subcutaneous | SC mistaken as SL (sublingual); SQ mistaken as “5 every;” the “q” in “sub q” has been mistaken as “every” (e.g., a heparin dose ordered “sub q 2 hours before surgery” misunderstood as every 2 hours before surgery) | Use “subcut” or “subcutaneously” |
| ss | Sliding scale (insulin) or ½ (apothecary) | Mistaken as “55” | Spell out “sliding scale;” use “one-half” or “½” |
| SSRI | Sliding scale regular insulin | Mistaken as selective-serotonin reuptake inhibitor | Spell out “sliding scale (insulin)” |
| SSI | Sliding scale insulin | Mistaken as Strong Solution of Iodine (Lugol’s) | Spell out “sliding scale (insulin)” |
| i/d | One daily | Mistaken as “tid” | Use “1 daily” |
| TIW or tiw | 3 times a week | Mistaken as “3 times a day” or “twice in a week” | Use “3 times weekly” |
| U or u** | Unit | Mistaken as the number 0 or 4, causing a 10-fold overdose or greater (e.g., 4U seen as “40” or 4u seen as “44”); mistaken as “cc” so dose given in volume instead of units (e.g., 4u seen as 4cc) | Use “unit” |
| UD | As directed (“ut dictum”) | Mistaken as unit dose (e.g., diltiazem 125 mg IV infusion “UD” misin- terpreted as meaning to give the entire infusion as a unit [bolus] dose) | Use “as directed” |
Error-Prone Dose Designations | |||
|---|---|---|---|
Dose Designations and Other Information | Intended Meaning | Misinterpretation | Correction |
| Trailing zero after decimal point (e.g., 1.0 mg)** | 1 mg | Mistaken as 10 mg if the decimal point is not seen | Do not use trailing zeros for doses expressed in whole numbers |
| “Naked” decimal point (e.g., .5 mg)** | 0.5 mg | Mistaken as 5 mg if the decimal point is not seen | Use zero before a decimal point when the dose is less than a whole unit |
| Abbreviations such as mg. or mL. with a period following the abbreviation | mg mL | The period is unnecessary and could be mistaken as the number 1 if written poorly | Use mg, mL, etc. without a terminal period |
| Drug name and dose run together (especially problematic for drug names that end in “l” such as Inderal40 mg; Tegretol300 mg) | Inderal 40 mg, Tegretol 300 mg | Mistaken as Inderal 140 mg, Mistaken as Tegretol 1300 mg | Place adequate space between the drug name, dose, and unit of measure |
| Numerical dose and unit of measure run together (e.g., 10mg, 100mL) | 10 mg, 100 mL | The “m” is sometimes mistaken as a zero or two zeros, risking a 10- to 100-fold overdose | Place adequate space between the dose and unit of measure |
| Large doses without properly placed commas (e.g., 100000 units; 1000000 units) | 100,000 units; 1,000,000 units | 100000 has been mistaken as 10,000 or 1,000,000; 1000000 has been mistaken as 100,000 | Use commas for dosing units at or above 1,000, or use words such as 100 "thousand" or 1 "million" to improve readability |
Error-Prone Drug Name Abbreviations | |||
|---|---|---|---|
Drug Name Abbreviations | Intended Meaning | Misinterpretation | Correction |
| To avoid confusion, do not abbreviate drug names when communicating medical information. Examples of drug name abbreviations involved in medication errors include: | |||
| APAP | acetaminophen | Not recognized as acetaminophen | Use complete drug name |
| ARA A | vidarabine | Mistaken as cytarabine (ARA C) | Use complete drug name |
| AZT | zidovudine (Retrovir) | Mistaken as azathioprine or aztreonam | Use complete drug name |
| CPZ | Compazine (prochlorperazine) | Mistaken as chlorpromazine | Use complete drug name |
| DPT | Demerol-Phenergan-Thorazine | Mistaken as diphtheria-pertussis-tetanus (vaccine) | Use complete drug name |
| DTO | Diluted tincture of opium, or deodorized tincture of opium (Paregoric) | Mistaken as tincture of opium | Use complete drug name |
| HCl | hydrochloric acid or hydrochloride | Mistaken as potassium chloride (The “H” is misinterpreted as “K”) | Use complete drug name unless expressed as a salt of a drug |
| HCT | hydrocortisone | Mistaken as hydrochlorothiazide | Use complete drug name |
| HCTZ | hydrochlorothiazide | Mistaken as hydrocortisone (seen as HCT250 mg) | Use complete drug name |
| MgSO4** | magnesium sulfate | Mistaken as morphine sulfate | Use complete drug name |
| MS, MSO4** | morphine sulfate | Mistaken as magnesium sulfate | Use complete drug name |
| MTX | methotrexate | Mistaken as mitoxantrone | Use complete drug name |
| NoAC | novel/new oral anticoagulant | No anticoagulant | Use complete drug name |
| PCA | procainamide | Mistaken as patient controlled analgesia | Use complete drug name |
| PTU | propylthiouracil | Mistaken as mercaptopurine | Use complete drug name |
| T3 | Tylenol with codeine No. 3 | Mistaken as liothyronine | Use complete drug name |
| TAC | triamcinolone | Mistaken as tetracaine, Adrenalin, cocaine | Use complete drug name |
| TNK | TNKase | Mistaken as “TPA” | Use complete drug name |
| TPA or tPA | tissue plasminogen activator, Activase (alteplase) | Mistaken as TNKase (tenecteplase), or less often as another tissue plasminogen activator, Retavase (retaplase) | Use complete drug name |
| ZnSO4 | zinc sulfate | Mistaken as morphine sulfate | Use complete drug name |
Error-Prone Stemmed Drug Names | |||
|---|---|---|---|
Stemmed Drug Names | Intended Meaning | Misinterpretation | Correction |
| “Nitro” drip | nitroglycerin infusion | Mistaken as sodium nitroprusside infusion | Use complete drug name |
| “Norflox” | norfloxacin | Mistaken as Norflex | Use complete drug name |
| “IV Vanc” | intravenous vancomycin | Mistaken as Invanz | Use complete drug name |
Error-Prone Symbols | |||
|---|---|---|---|
Symbols | Intended Meaning | Misinterpretation | Correction |
| Dram | Symbol for dram mistaken as “3” | Use the metric system |
| Minim | Symbol for minim mistaken as “mL” | Use the metric system | |
| x3d | For three days | Mistaken as “3 doses” | Use “for three days” |
| > and < | More than and less than | Mistaken as opposite of intended; mistakenly use incorrect symbol; “< 10” mistaken as “40” | Use “more than” or “less than” |
| / (slash mark) | Separates two doses or indicates “per” | Mistaken as the number 1 (e.g., “25 units/10 units” misread as “25 units and 110” units) | Use “per” rather than a slash mark to separate doses |
| @ | At | Mistaken as “2” | Use “at” |
| & | And | Mistaken as “2” | Use “and” |
| + | Plus or and | Mistaken as “4” | Use “and” |
| ° | Hour | Mistaken as a zero (e.g., q2° seen as q 20) | Use “hr,” “h,” or “hour” |
| Φ or Ø | zero, null sign | Mistaken as numerals 4, 6, 8, and 9 | Use 0 or zero, or describe intent using whole words |
High-Alert Medications and Black Box Warnings
High-alert (or high-hazard) medications are those that are “most likely to cause significant harm to the patient even when used as intended.” However, some of these medications also have a higher volume of use than other medications. Though medication mishaps with these high-alert drugs are no more frequent than with other drugs, the consequences can be devastating (USDVA, 2015a; IHI, 2012).
High-alert drugs fall into as many as 19 categories and improved management of all of them is important, but four categories are more frequently associated with harm:
- Anticoagulants
- Narcotics and opiates
- Insulins
- Sedatives (IHI, 2012)
The types of harm most frequently associated with these drugs include hypotension, bleeding, hypoglycemia, delirium, lethargy, and over-sedation (IHI, 2012).
The FDA’s Black Box Warning System alerts healthcare providers and consumers to drugs with increased risks for those taking them. These warnings are meant to be the strongest labeling requirement for drugs and drug products that can have serious adverse reactions or potential safety hazards, especially those that may result in death or injury. The black box—a heavy black line surrounding the warning—appears on the prescription label to alert patient and provider about safety concerns.
The FDA (www.fda.gov) does not issue an itemed list of these high-alert drugs but does provide detailed information about each of them, which is constantly updated with new information. Some commonly used black box–warning drugs include heparin, warfarin, insulin, Avandia, Ritalin, estrogen-containing contraceptives, and most antidepressants. Although a large percentage of patients are prescribed medications with black box warnings, many do not understand the warnings or receive the advised laboratory monitoring (Hughes & Blegen, 2008).
Healthcare-Associated Infections (HAIs)
Healthcare-associated infections (HAIs) are some of the most common complications associated with hospital care in the United States.
In the most recent HAI prevalence survey using 2015 data, researchers from the CDC found that about 1 in 31 hospital patients had at least one healthcare-associated infection. Patients in the 2015 survey were 16% less likely than patients in the 2011 survey to have an HAI. There were an estimated 687,000 HAIs in U.S. acute care hospitals in 2015 and about 72,000 hospital patients with HAIs died during their hospitalization (AHRQ, 2019a; CDC, 2018b).
A 2013 study found that just five types of infections account for some $9.8 billion annually (Zimlichman et al., 2013) and the costs of HAIs are everywhere into the multi-millions of dollars—for events that are widely known to be largely preventable.
The National Action Plan to Prevent Healthcare-Associated Infections: Road Map to Elimination by the federal Office of Disease Prevention and Health Promotion (ODPHP) and a broad consortium of other federal agencies, including the U.S. Department of Health and Human Services (HHS) and CDC, adopted the following six priority areas with reduction targets:
- Catheter-associated urinary tract infection
- Clostridium difficile Infection (now Clostridioides difficile)
- Central line–associated bloodstream infection
- MRSA (Methicillin-resistant Staphylococcus aureus) infection
- Surgical site infection
- Ventilator-associated pneumonia (now ventilator-associated events) (US ODPHP, 2013)
Surgical Site Infections (SSIs)
Surgical site infections (SSIs) are those that occur after surgery in the part of the body where the surgery took place. SSIs can sometimes be superficial, involving only the skin, but others are more serious and can involve tissues under the skin, organs, or implanted material.
Common symptoms of an SSI include:
- Redness and pain near the surgical wound
- Drainage of cloudy fluid from the surgical wound
- Fever (CDC, 2010)
SSIs are the most common hospital-acquired infection, according to the CDC, accounting for more than 30% of all inpatient HAIs. While there have been advances in infection control practices and decreases in some SSIs, they remain a substantial cause of morbidity, prolonged hospitalization, and death. In addition, they are the mostly costly HAI type, with an estimated annual cost of $3.3 billion, and are associated with 1 million additional inpatient-days annually (NHSN, 2019).
SSIs are not only a national issue, but a local one as well. A 2012 study of 851 patients at nine hospitals in Jacksonville, Florida, found 51 had HAIs, 18 with surgical site infections. These accounted for the largest type of HAI in the study, or 35% among the patients with HAIs (Magill et al., 2012). As noted earlier, the most recent data for Florida shows no significant change from 2015 to 2016 in SSIs related to abdominal hysterectomy surgery but a significant decrease in SSIs associated with colon surgery (CDC, 2018).
Preventing SSIs
New detailed guidelines for prevention of SSIs were released by the CDC in 2017. They and related information are contained in the National Healthcare Safety Network (NHSN) Patient Safety Component Manual updated in January 2019 and in the JAMA Surgery Special Communication on the guidelines issued in August 2017 (see NHSN, 2019, and Berríos-Torres et al., 2017, in Resources).
Central Line–Associated Bloodstream Infections
A central line (also known as a central venous catheter) is a catheter (tube) often placed in a large vein in the neck, chest, or groin to give medication or fluids or to collect blood for medical tests. Unlike more familiar IVs, central lines access a major vein that is close to the heart and can remain in place for weeks or months and be much more likely to cause serious infection. Central lines are commonly used in intensive care units but are used in other situations as well (CDC, 2011).
A central line-associated bloodstream infection (CLABSI) is a serious infection that occurs when germs (usually bacteria or viruses) enter the bloodstream through the central line. They result in thousands of deaths each year and billions of dollars in added costs to the U.S. healthcare system, yet these infections are preventable (CDC, 2016, 2011).
Healthcare providers follow a strict protocol when inserting the line to make sure it remains sterile. In addition, they use stringent infection control practices each time they check the line or change the dressing. Patients who get a CLABSI have a fever and might also have red skin and soreness around the central line. If this happens, tests must be done to determine if there is an infection present (CDC, 2011).
The good news is that prevention measures of CLABSIs are having an impact. The CDC’s 2016 HAI Progress Report, based on 2014 data, shows a significant decrease in CLABSIs between 2013 and 2014. Florida also saw a decrease in the same time period (CDC, 2016).
Preventing CLABSIs
Healthcare providers can take the following steps to help prevent CLABSIs:
- Perform hand hygiene.
- Apply appropriate skin antiseptic.
- Ensure that the skin prep agent has completely dried before inserting the central line.
- Use all five maximal sterile barrier precautions:
- Sterile gloves
- Sterile gown
- Cap
- Mask
- Large sterile drape
- Once the central line is in place:
- Follow recommended central line maintenance practices.
- Wash their hands with soap and water or an alcohol-based hand rub before and after touching the line.
- Remove a central line as soon as it is no longer needed. The sooner a catheter is removed, the less likely the chance of infection. (CDC, 2011)
More detailed information and guidelines on CLASBI prevention is available from the CDC, the Joint Commission (2012), and the National Healthcare Safety Network (NHSN, 2019), and should be a part of all healthcare facility training protocols.
Ventilator-Associated Events (VAE) and Ventilator-Associated Pneumonia (VAP)
In 2011 an estimated 157,000 healthcare-associated pneumonias occurred in acute care hospitals in United States; 39% of these pneumonias were ventilator-associated (VAP). Patients receiving invasive mechanical ventilation are at risk for numerous complications, including pneumonia.
Ventilator-associated pneumonia (VAP) and other healthcare-associated pneumonias are important common healthcare-associated infections, but national surveillance for VAP has long been a challenge because of the lack of objective reliable definitions. Due to these challenges, in January 2013 the National Healthcare Safety Network (NHSN) replaced surveillance for ventilator-associated pneumonia (VAP) in adult inpatient locations with surveillance for ventilator-associated events (VAE). (NHSN, 2019).
Mechanical ventilation is an essential, life-saving therapy for patients with critical illness and respiratory failure. Studies have estimated that more than 300,000 patients receive mechanical ventilation in the United States each year. These patients are at high risk for complications and poor outcomes, including death. Ventilator-associated pneumonia (VAP), sepsis, acute respiratory distress syndrome (ARDS), pulmonary embolism, barotrauma, and pulmonary edema are among the complications that can occur in patients receiving mechanical ventilation; such complications can lead to longer duration of mechanical ventilation, longer stays in the ICU and hospital, increased healthcare costs, and increased risk of disability and death (NHSN, 2019).
Preventing VAP
To prevent ventilator-associated pneumonia, healthcare providers can do the following things:
- Keep the head of the patient’s bed raised between 30 and 45 degrees unless other medical conditions do not allow this.
- Check the patient’s ability to breathe on own every day so that the patient can be taken off of the ventilator as soon as possible.
- Clean hands with soap and water or an alcohol-based hand rub before and after touching the patient or the ventilator.
- Clean the inside of the patient’s mouth on a regular basis.
- Clean or replace equipment between use on different patients. (CDC, 2010a)
Detailed current guidance on diagnosing and managing VAP and VAEs is available from NHSN (2019).
Catheter-Associated Urinary Tract Infections
Urinary tract infections (UTIs)—infection involving any of the organs or structures of the urinary tract, including urethra, bladder, ureters, and kidneys—are the most common type of healthcare-associated infection reported to the National Healthcare Safety Network (NHSN). Some of the common symptoms of a urinary tract infection are burning or pain in the lower abdomen (that is, below the stomach), fever, burning during urination, or an increase in the frequency of urination.
Among UTIs acquired in the hospital, approximately 75% are associated with a urinary catheter. An indwelling urinary catheter is a drainage tube that is inserted into the urinary bladder through the urethra, is left in place, and is connected to a closed collection system.
Between 15% and 25% of hospitalized patients receive urinary catheters during their hospital stay, and the most important risk factor for developing a catheter-associated UTI (CAUTI) is prolonged use of the urinary catheter. Therefore, catheters should only be used for appropriate indications and should be removed as soon as they are no longer needed. (CDC, 2017a, 2015a; Lo et al., 2014).
Preventing CAUTIs
The CDC recommends that healthcare practitioners utilize the Guideline for Prevention of Catheter-Associated Urinary Tract Infections, 2009 (updated 2017) found at https://www.cdc.gov/infectioncontrol/guidelines/CAUTI/index.html. The guideline emphasizes the proper use, insertion, and maintenance of urinary catheters in various healthcare settings. It also presents effective quality improvement programs that healthcare facilities can use to prevent CAUTIs. Links to additional guidelines are listed here.
In a December 2018 report, the AHRQ noted that Tampa General Hospital had lowered the CAUTI rate attributed to the emergency department by 75% and reduced the department’s indwelling urinary catheter utilization ratio by 23%. This was accomplished by use of a broadly supported program that integrated tools from AHRQ (AHRQ, 2018e).
Hospital-Onset Clostridioides difficile Infections
Clostridioides difficile (formerly Clostridium difficile) is a bacterium that causes diarrhea that can be life threatening and colitis, an inflammation of the colon. It is usually a side effect of taking antibiotics. Clostridioides difficile is also called C. difficile, C. diff, CDI (C. diff infection), and CDAD (Clostridioides difficile-associated disease).
C. difficile can easily spread from person to person and is a major health threat. A CDC study in 2015 found that it caused almost half a million infections among patients in the United States in a single year. An estimated 15,000 deaths are directly attributable to C. difficile infections (CDC, 2019, 2019a).
Poor antibiotic prescribing practices put patients at risk for C. diff infections (CDI). More than half of all hospitalized patients get an antibiotic at some point during their hospital stay, but studies have shown that 30% to 50% of antibiotics prescribed in hospitals are unnecessary or incorrect (CDC, 2019).
Additional risk factors include:
- Being 65 or older
- Complicated medical care and extended stays in settings like hospitals and nursing homes
- Certain antibiotics, such as fluoroquinolones
- A weakened immune system
- Previous infection with C. diff or known exposure to the germs (CDC, 2019, 2019a, 2018c)
Symptoms, which might start within a few days or even several weeks after a patient begins taking antibiotics, include:
- Diarrhea including loose, watery stools or frequent bowel movements for several days
- Fever
- Stomach tenderness or pain
- Loss of appetite
- Nausea
C. difficile is carried from person to person in feces. Any surface, device, or material (eg, toilets, bathtubs, electronic rectal thermometers) that becomes contaminated with feces may serve as a reservoir for the spores. C. difficile spores are often transferred to patients via the hands of healthcare personnel who have touched a contaminated surface or item. C. difficile can live for long periods on surfaces (CDC, 2018c).
While C. difficile can be deadly, protocols put in place by multiple public and private healthcare organizations are having an effect on the spread of the disease. The National and State Healthcare-Associated Infections Progress Report showed an 8% decrease in hospital-onset C. difficile infections between 2011 and 2014 (CDC, 2016a).
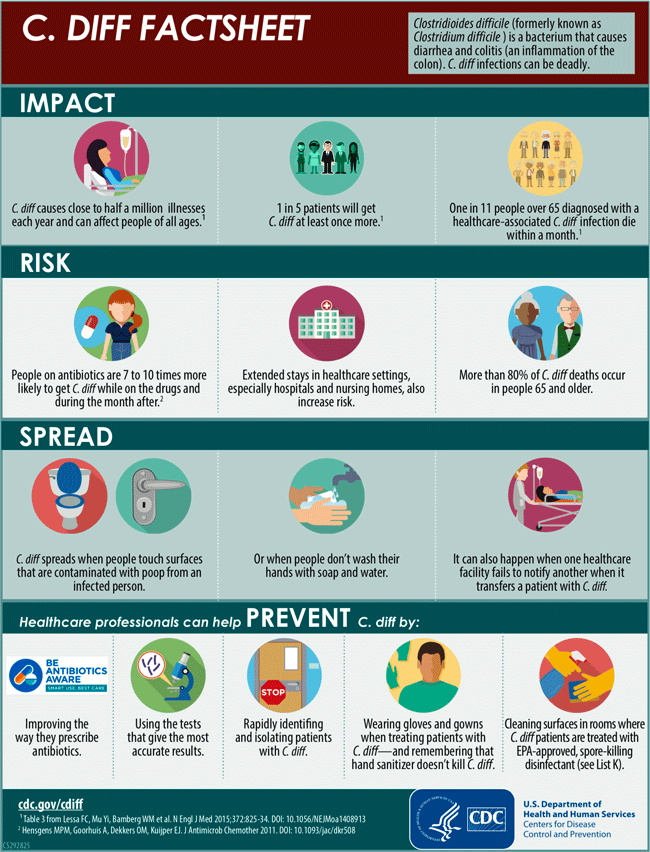
C. Diff Factsheet available at https://www.cdc.gov/cdiff/pdf/Cdiff-Factsheet-508.pdf
Preventing C. difficile Infections
To prevent C. difficile infections, healthcare providers should:
- Use antibiotics judiciously.
- Use contact precautions for patients with known or suspected CDI:
- Place these patients in private rooms. If private rooms are not available, they can be placed in rooms (cohorted) with other CDI patients.
- Use gloves when entering patients’ rooms and during patient care.
- Perform hand hygiene after removing gloves.
- Because alcohol does not kill C. diff spores, use of soap and water is more effective than alcohol-based hand rubs. However, early experimental data suggest that, even using soap and water, the removal of C. diff spores is more challenging than the removal or inactivation of other common pathogens.
- Using gloves to prevent hand contamination remains the cornerstone for preventing C. diff transmission via the hands of healthcare personnel; any theoretical benefit from instituting soap and water must be balanced against the potential for decreased compliance resulting from a more complex hand hygiene message.
- If your institution experiences an outbreak, consider using only soap and water for hand hygiene after removing gloves while caring for patients with CDI.
- Use gowns when entering patients’ rooms and during patient care.
- Dedicate or perform cleaning of any shared medical equipment.
- Continue these precautions until diarrhea ceases.
- Because C. diff-infected patients continue to shed the organism for a number of days following cessation of diarrhea, some institutions routinely continue isolation and contact precautions for either several days beyond symptom resolution or until discharge, depending upon the type of setting and average length of stay.
- Implement an environmental cleaning and disinfection strategy.
- Ensure adequate cleaning and disinfection of environmental surfaces and reusable devices, especially items likely to be contaminated with feces and surfaces that are touched frequently.
- Ensure daily and terminal cleaning of patient rooms.
- Use an Environmental Protection Agency (EPA)-registered disinfectant with a sporicidal claim for environmental surface disinfection after cleaning in accordance with label instructions. (Note: Only hospital surface disinfectants listed on EPA’s List K are registered as effective against C. diff spores.)
- Follow the manufacturer’s instructions for disinfection of endoscopes and other devices.
- Ensure adequate cleaning and disinfection of environmental surfaces and reusable devices, especially items likely to be contaminated with feces and surfaces that are touched frequently.
Recommended infection control practices in long-term care and home health settings are similar to those practices taken in traditional healthcare settings (CDC, 2018c).
Antibiotic/Antimicrobial Resistance (AR/AMR)
Antibiotic resistance is one of the biggest public health challenges of our time. Antibiotic resistance happens when germs like bacteria and fungi develop the ability to defeat the drugs designed to kill them.
Each year in the United States, at least 2 million people get an antibiotic-resistant infection, and at least 23,000 people die. Antibiotic resistance has the potential to affect people at any stage of life, as well as the healthcare, veterinary, and agriculture industries, making it one of the world’s most urgent public health problems.
No one can completely avoid the risk of resistant infections, but some people are at greater risk than others (eg, people with chronic illnesses). If antibiotics lose their effectiveness, then we lose the ability to treat infections and control public health threats.
Many medical advances are dependent on the ability to fight infections using antibiotics, including joint replacements, organ transplants, cancer therapy, and treatment of chronic diseases like diabetes, asthma, and rheumatoid arthritis (CDC, 2018d).
Methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococcus spp. (VRE), and certain gram-negative bacilli have increased in prevalence in U.S. hospitals over the last three decades, and have important implications for patient safety. There is concern about these multidrug-resistant organisms (MDROs) as options for treating patients with these infections are often extremely limited, and MDRO infections are associated with increased lengths of stay, costs, and mortality. Many of these traits have also been observed for Clostridioides difficile infection (CDI) (NHSN, 2019).
In the 2013 CDC report Antibiotic Resistance Threats in the United States, 2013 (AR Threats Report) the threat level of certain MDROs was different than has been identified currently. The CDC is planning to release an updated Threats Report in Fall 2019. In the meantime, the Patient Safety Component Manual (NHSN, 2019) and the CDC AR/AMR website present more current information, and the guidelines for managing MDROs updated in 2017 (CDC, 2017c) are available here.
Methicillin-Resistant Staphylococcus aureus (MRSA)
Methicillin-resistant Staphylococcus aureus (MRSA) is a staph bacterium that is resistant to many antibiotics. In a healthcare setting, such as a hospital or nursing home, MRSA can cause severe problems such as bloodstream infections, pneumonia, and surgical site infections. If not treated quickly, MRSA infections can cause sepsis and death (CDC, 2018e).
MRSA is usually spread by direct contact with an infected wound or from contaminated hands, usually those of healthcare providers. Also, people who carry MRSA but do not have signs of infection can spread the bacteria to others. CDC recommends the use of Contact Precautions (CP) in inpatient acute care settings for patients known to be colonized or infected with epidemiologically important MDROs including MRSA (CDC, 2018e).
MRSA is preventable, and the CDC offers numerous guidelines and tools for healthcare professionals on its website, https://www.cdc.gov/mrsa/healthcare/clinicians/materials-hcp/index.html.
Source: CDC, 2018d.
Vancomycin-resistant Enterococcus spp. (VRE)
Vancomycin-resistant Enterococci (VRE) are specific types of antimicrobial-resistant bacteria that are resistant to vancomycin, the drug often used to treat infections caused by enterococci. Enterococi are bacteria that are normally present in the human intestines and in the female genital tract and are often found in the environment. These bacteria can sometimes cause infections and most vancomycin-resistant Enterococci infections occur in hospitals (CDC, 2011a).
VRE can live in the human intestines and female genital tract without causing disease (this is often called colonization). However, sometimes VRE can cause infections of the urinary tract, the bloodstream, or of wounds associated with catheters or surgical procedures. (CDC, 2011a).
VRE is often spread from person to person by the contaminated hands of caregivers who have had contact with people with VRE or contaminated surfaces, or it can be spread directly to a person from a contaminated surface. It is not spread through the air by coughing or sneezing (CDC, 2011a).
Hospitalized patients are at an increased risk of VRE infection, especially those:
- Receiving antibiotic treatment for long periods of time
- With weakened immune systems
- Who have had surgical procedures, such as abdominal or chest surgery
- Who have medical devices, such as catheters, in place for some time
- Who are colonized with VRE (CDC, 2011a)
Hand Hygiene: Best Practice to Prevent HAIs
Healthcare-associated infections—as dangerous and even deadly as they are—can be mitigated by one of the simplest methods of infection control, good hand hygiene.
Practicing hand hygiene is a simple yet effective way to prevent infections. Cleaning your hands can prevent the spread of germs, including those that are resistant to antibiotics and are becoming difficult, if not impossible, to treat. On average, healthcare providers clean their hands less than half of the times they should. Healthcare providers might need to clean their hands as many as 100 times per 12-hour shift, depending on the number of patients and intensity of care. Know what it takes to keep your patients safe! (CDC, 2018f, 2016b).
Healthcare providers should practice hand hygiene at key points in time to disrupt the transmission of microorganisms to patients. These include:
- Before eating
- Before and after having direct contact with a patient’s intact skin (taking a pulse or blood pressure, performing physical examinations, lifting the patient in bed)
- After contact with blood, body fluids or excretions, mucous membranes, non-intact skin, or wound dressings
- After contact with inanimate objects (including medical equipment) in the immediate vicinity of the patient
- If hands will be moving from a contaminated-body site to a clean-body site during patient care
- After glove removal
- After using a restroom (CDC, 2018g)
Two Methods for Sentinel Event
Which is preferred: alcohol-based hand sanitizer or washing with soap and water? In 2019, CDC offered the following advice:
- Alcohol-based hand sanitizers are the most effective products for reducing the number of germs on the hands of healthcare providers. Antiseptic soaps and detergents are the next most effective and non-antimicrobial soaps are the least effective.
- When hands are not visibly dirty, alcohol-based hand sanitizers are the preferred method for cleaning your hands in the healthcare setting.
- Soap and water are recommended for cleaning visibly dirty hands.
During routine patient care:
Wash with soap and water
- When hands are visiUse an alcohol-based hand sanitizerbly dirty
- After known or suspected exposure to Clostridioides difficile if your facility is experiencing an outbreak or higher endemic rates
- After known or suspected exposure to patients with infectious diarrhea during norovirus outbreaks
- If exposure to Bacillus anthracis is suspected or proven
- Before eating
- After using a restroom
Use an alcohol-based hand sanitizer
- For everything else (CDC, 2018g)
Additional guidelines for hand washing techniques to use in both situations are available on the CDC website here.
Surgical Errors
According to an AHRQ-supported study, wrong-site surgery occurred at a rate of approximately 1 per 113,000 operations between 1985 and 2004. In July 2004 the Joint Commission enacted the Universal Protocol, which requires performing a time-out prior to beginning surgery, a practice that has been shown to improve teamwork and decrease the overall risk of wrong-site surgery. Developed through expert consensus on principles and steps for preventing wrong-site, wrong-procedure, and wrong-person surgery, the Universal Protocol applies to all accredited hospitals, ambulatory care, and office-based surgery facilities.
Wrong-site, wrong-procedure, and wrong-patient errors are all now considered never events (medical errors that should never occur) by the National Quality Forum (NQF) and sentinel events (events resulting in death, permanent harm, or severe temporary harm and intervention required to sustain life) by the Joint Commission. CMS has not reimbursed healthcare providers for any costs associated with these surgical errors since 2009 (PSNet 2019; 2003; Joint Commission, 2017).
In 2011, NQF and other agencies added “unintended retention of a foreign object in a patient after surgery or other procedure” to its list of never events for surgeries, and this is also among the hospital-acquired conditions for which CMS will not reimburse (CMS, 2015).
Another serious, not to say disastrous, case of medical error is the following.
A 53-year-old man presented to Hospital A with abdominal pain and hematuria. Computed tomography (CT) imaging revealed a suspected renal cell carcinoma in the right kidney. He was transferred to Hospital B for surgical management.
All of the medical records from Hospital A documented a left-sided tumor—the wrong side. The CT scan from Hospital A was not available at the time of the transfer and repeat imaging was not obtained by the providers at Hospital B.
At the time of surgery, the surgeon was asked if the absence of an available image should preclude progressing with the surgery. He decided to proceed and, based on the available information, removed the left kidney.
The day following the surgery, the pathologist contacted the surgeon to report no evidence of cancer. The surgeon then reviewed the initial CT scan and realized his mistake. The patient underwent a second surgical procedure to remove the right kidney (which was found to have renal cell carcinoma). Having lost both kidneys, the patient was then dependent on dialysis, and because of the cancer, he was not a candidate for kidney transplant.
Discussion
A total of four errors resulted in this sentinel event. The first was a documentation error on the medical records from Hospital A (identifying the tumor on the wrong side), which most likely originated from the original CT report.
The second error occurred during the patient transfer, when only the records, but not the imaging, accompanied the patient.
The third error occurred as the patient was posted for the surgical suite without preoperative imaging. In most cases, when imaging does not accompany a patient in transfer, the patient is reimaged to confirm the diagnosis and for preoperative planning, particularly if there is no emergent reason to proceed. These three errors occurred before the patient was rolled into the surgical suite. At this point, though, the error still could have been prevented.
The fourth error occurred once in the operating room—implementation of the Universal Protocol could have been effective, but only if completely implemented by the surgical team. The protocol suggests having the labeled radiology images present and available in the operating room at the time of the surgery. Once the surgeon decided he did not need the imaging to proceed with surgical treatment, the proverbial cat was out of the bag. This is because the Universal Protocol does not differentiate between types of cases requiring imaging and those that do not. Many surgical cases do not require preoperative imaging, and the presence or absence of imaging is left to the discretion of the surgeon. While this flexibility may be useful at times, it can give rise to human error, as it did in this case.
Source: AHRQ, 2015. Commentary by John G. DeVine, MD.
Universal Protocol: Best Practice to Prevent Surgical Errors
To address the problem of preventable surgical errors, the Joint Commission issued its Universal Protocol on July 1, 2004, and it has become a mandatory patient safety standard in healthcare ever since. The protocol consists of the following three components:
- A pre-procedure verification process
- Surgical site marking
- Surgical “time out” immediately prior to starting the procedure
The surgical site must be marked and visible after prepping and draping of the patient. Using the surgical time-out as “reflective pause or a preoperative briefing” involves the surgeons, anesthesiologists, nurses, quality control specialists, and administrators. Recent studies show the surgical time-out is an effective quality control measure (Altpeter et al, 2007; Stahel et al., 2009; Joint Commission, 2019a).
Laboratory Errors
An estimated 13 billion laboratory tests are performed each year in over 250,000 certified laboratories in the United States. Laboratory services account for 2.3% of healthcare expenditures (2% of Medicare expenditures), and these services are integral to patient care (AACC, 2015).
In fact, emergency departments order clinical laboratory tests in more than 41% of all visits, family physicians order tests in 29% of visits, and general internists in 38% of visits (Epner et al., 2013). Up to 70% of clinician decisions are influenced by laboratory tests, indicating that clinical laboratories have an important role in helping to reduce avoidable medical errors and improve both patient safety and outcomes (Tieman, 2017).
CLIA and Laboratory Errors
Thirty years ago, the CDC, CMS, and FDA developed the Clinical Laboratory Improvement Amendments of 1988 (CLIA), a sweeping set of regulations for all U.S. facilities or sites that test human specimens for health assessment or to diagnose, prevent, or treat disease. A critical component of these regulations was quality control. Final regulations were published in 2003 (CDC, 2018h).
CLIA urges laboratories to develop an individualized quality control plan addressing five areas for assessing risk: specimen, test system, reagents, environment, and testing personnel (CLIA, 2014).
Laboratory testing is often broken into three stages: pre-analytic, analytic, and post-analytic (CLIA, 2014). Studies have shown nearly 70% of errors occur in the pre-analytic phase, encompassing test requests, patient and specimen identification, specimen collection, transport, accessioning, or processing (Osborne, 2018; Tieman, 2017; Kaushik & Green, 2014).
Poor communication between laboratory and healthcare professionals is the main issue affecting quality in the pre- and post-analytic phases, and researchers note few in either group receive specific training in good communication techniques. Issues of test choice, patient information, specimen adequacy (in pre-phase), and values and interpretation (in post-phase) can involve many different healthcare professionals, and poor communication among them can result in errors, patient harm, and “inefficient and ineffective use of healthcare resources.” Errors also occur when clinicians choose and order tests; during specimen collection, including mislabeling, improper collection, and specimen contamination; in laboratory processing; and in results analysis and reporting (Wolcott et al., 2008).
Impact of Waived Tests on Laboratory Testing
As part of CLIA, some simple low-risk tests were waived from laboratory quality requirements and performed with no routine regulatory oversight in physicians' offices and various other locations.
In 1993, CLIA waived nine such tests; today there are more than 5,400 waived test systems and 119 analytes, according to the Commission on Office Laboratory Accreditation (COLA), an independent laboratory accreditation agency recognized by both CMS and the Joint Commission (COLA, 2015).
Although by law waived tests should have insignificant risk for erroneous results, these tests are not completely error-proof and are not always used in settings that employ a systems approach to quality and patient safety. Errors can occur anywhere in the testing process, particularly when the manufacturer's instructions are not followed, and when testing personnel are not familiar with all aspects of the test system and how testing is integrated into the facility's workflow (CDC, 2018j; COLA, 2015).
Although data have not been systematically collected on patient outcomes with waived testing, adverse events can occur. Some waived tests have potential for serious health impacts if performed incorrectly. For example, results from waived tests can be used to adjust medication dosages, such as prothrombin time testing in patients undergoing anticoagulant therapy or glucose monitoring in diabetics. In addition, erroneous results from diagnostic tests, such as those for human immunodeficiency virus (HIV) antibody, can have unintended consequences (CDC, 2018j).
Preventing Laboratory Errors
Although laboratory medicine has had long a history of formalized approaches of mitigating errors, most laboratory quality control programs focus on reducing testing errors as opposed to a systems approach preventing diagnostic harm to patients (Epner et al., 2013).
Several studies are reviewing an outcomes-based approach to reducing and preventing errors. Epner and colleagues suggest five causes that, taken together, may explain all important sources of diagnostic error and harm related to the testing process (see box below). “While occurrences of the five causes will not always result in diagnostic error, patient harm related to diagnostic testing is highly likely to stem from one of these five causes” (Epner et al., 2013).
Five Causes of Diagnostic Error and Harm
Five causes taxonomy of testing-related diagnostic error:
- An inappropriate test is ordered.
- An appropriate test is not ordered.
- An appropriate test result is misapplied.
- An appropriate test is ordered, but a delay occurs somewhere in the total testing process.
- The result of an appropriately ordered test is inaccurate.
Source: Epner, Gans, & Graber, 2013.
Helping to reduce errors in lab testing is another area where education and personal advocacy can improve outcomes. It has been observed that many people have the tendency to think that “laboratory tests are always correct and useful.” This is not true and all tests have their limitations. A consumer’s best protection is to be informed: know where problems can arise and what to ask or do to help avoid those problems. This includes the following:
- Make sure it is the right test: ask why it is or is not being ordered and what the results will add to your care, are there any risks, and why has your doctor ordered the test.
- Use the right sample: if the test relies on a patient provided sample, be sure you understand how to correctly collect the sample, what to put it in, and how to handle it after you collect it.
- Understand that errors can happen even under the best of conditions: your best defense is using a reputable lab that employs verified methods, and understanding the test’s performance. If the results don’t make sense, ask questions.
- Understand your results in context: use trusted information sources for details on the meaning and use of your results, and, again, ask questions! (Haymond, 2016)
Patient Falls
No clinician working alone, regardless of how talented, can prevent all falls. Rather, fall prevention requires the active engagement of many individuals, including the multiple disciplines and teams involved in caring for the patient.
AHRQ, 2013
Preventing Falls in Hospitals
According to AHRQ a patient fall is defined as “an unplanned descent to the floor with or without injury to the patient.” Such falls can result in fractures, lacerations, or internal bleeding, requiring additional healthcare. Research has shown that close to one-third of falls are preventable (AHRQ, 2018b).
Falls happen for a number of reasons including:
- Person is weak, tired or ill.
- Person is not physically fit.
- Person may have problems seeing.
- Medicines may cause weakness, sleepiness, confusion or dizziness.
- Slippery or wet floors or stairs.
- Obstructed pathways.
- Darkness. (Joint Commission, 2018)
Treating fall injuries is very costly. In 2015, total medical costs for falls totaled more than $50 billion. Because the U.S. population is aging, both the number of falls and the costs to treat fall injuries are likely to rise. Every year 3 million older people are treated in emergency departments because of falls and over 800,000 patients are hospitalized because of a fall injury—most commonly broken hips and head injuries (CDC, 2017d, 2016c).
The average hospital cost for a fall injury is more than $30,000 and the costs go up with age. In 2015 total medical costs for falls were more than $50 billion and Medicare and Medicaid shouldered 75% of these costs (CDC, 2017d).
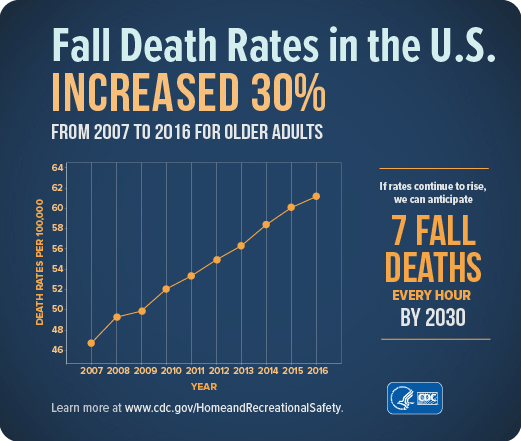
Source: CDC.
Preventing Patient Falls
Falls within care settings are especially concerning. The Joint Commission Center for Transforming Healthcare notes that hundreds of thousands of patients fall in hospitals every year and 30% to 35% experience an injury. The estimated average cost for a fall with injury is about $14,056 (JCC, 2019).
Hospital staff have a complex and potentially conflicting set of patient care goals. They need to treat the problem that prompted the patient's admission, keep the patient safe, and help the patient to maintain or recover physical and mental function. Thus, fall prevention must be balanced against other priorities (AHRQ, 2013).
Fall prevention involves managing a patient's underlying fall risk factors (eg., problems with walking and transfers, medication side effects, confusion, frequent toileting needs) while working within the hospital's physical design and environment. A number of practices have been shown to reduce the occurrence of falls, but these practices are not used systematically in all hospitals (AHRQ, 2013).
Fall prevention requires an interdisciplinary approach to care. Some aspects of fall prevention care are highly routinized, while others must be tailored to each patient's specific situation. Fall prevention requires the active engagement of all the multiple disciplines and teams involved in caring for the patient. This sort of coordination for high-quality prevention requires an organizational culture and operational practices that promote teamwork and communication, as well as individual expertise (AHRQ, 2013).
Fall prevention activities also need to be balanced with other considerations, such as minimizing restraints and maintaining patients' mobility, to provide the best possible care to the patient. Therefore, improvement in fall prevention requires a system focus to make needed changes (AHRQ, 2013).
What can individuals do to reduce their risk of falls? The Joint Commission provides guidelines targeted toward patients at home and in hospitals or nursing facilities.
At home guidelines include:
- Turn on the lights when you enter a room. Do not walk in the dark.
- Make sure your pathway is clear.
- Use the handrails on staircases.
- Sit in chairs that do not move and have arm rests to help when you sit down and stand up.
- Wear shoes that have firm, flat, non-slip soles. Do not wear shoes that do not have backs on them.
- Replace the rubber tips on canes and walkers when they become worn. (Joint Commission, 2018)
For in-patient settings guidelines include:
- Use your call button to ask for help getting out of bed if you feel unsteady.
- Ask for help going to the bathroom or walking around the room or in hallways.
- Wear non-slip socks or footwear.
- Lower the height of the bed and the side rails.
- Talk to your doctor if your medicine makes you sleepy, light-headed, sluggish or confused. Ask how to reduce these side effects or if you can take another medicine (Joint Commission, 2018).
Online Resource
Speak Up™ To Prevent Falls
https://www.jointcommission.org/topics/speak_up_reducing_your_risk_of_falling.aspx
Agency for Healthcare Research and Quality
The AHRQ’s text, Patient Safety and Quality: An Evidence-Based Handbook for Nurses, released in 2008, includes a chapter specific to patient falls in different care settings, with a discussion of fall risk assessment tools and prevention strategies (see table below) (Currie, 2008).
Recommendations for Acute and Long-Term Care | |
|---|---|
Evidence-based practice recommendations | Research implications |
| Fall Prevention | |
|
|
| Injury Prevention | |
|
|
Also, updated in 2018, AHRQ published Preventing Falls in Hospitals: A Toolkit for Improving Quality of Care, which discusses the development of a complete program for hospitals, including such practices as rounding protocols (AHRQ, 2018b).
Joint Commission Center for Transforming Healthcare
The Joint Commission released its Targeted Solutions Tool for Patient Falls with Injury, in August 2015. The tool is an innovative application that guides healthcare organizations through a step-by-step process to accurately measure their organization’s actual performance, identify their barriers to excellent performance, and direct them to proven solutions that are customized to address their particular barriers. According to the Commission, organizations that followed its standardized approach reduced the rate of patient falls by 35% and falls with injury by 62% (JCC, 2019).
Tens of thousands of patients fall in healthcare facilities every year and many of these falls result in moderate to severe injuries. Find out how the participants in the Center for Transforming Healthcare’s seventh project are working to keep patients safe from falls.
Keeping Patients Safe from Falls
Source: The Joint Commission (2012).
https://www.youtube.com/watch?v=pxwW88qTGqw
CDC’s STEADI: Older Adult Fall Prevention
CDC created the evidence-based STEADI (Stopping Elderly Accidents, Deaths, and Injuries) initiative to help healthcare providers incorporate fall prevention into routine care for older adults. STEADI provides screening tools, educational materials and resources, and online trainings for healthcare providers. Information can be accessed on the CDC’s STEADI website (CDC, 2016d).
Pressure Ulcers
Pressure ulcers, sometimes called decubitus ulcers, pressure sores, or bedsores, are areas of damaged skin caused by staying in one position for too long. They commonly form where bones are close to the skin, such as ankles, back, elbows, heels, and hips. Patients are at risk if they are bedridden, use a wheelchair, or are unable to change their position. Pressure sores can cause serious infections, some of which are life-threatening (MedLine Plus, 2018).
The Institute for Healthcare Improvement (IHI) notes that
Because muscle and subcutaneous tissue are more susceptible to pressure-induced injury than skin, pressure ulcers are often worse than their initial appearance. Pressure ulcers cause considerable harm to patients, hindering functional recovery, frequently causing pain and the development of serious infections. Pressure ulcers have also been associated with an extended length of stay, sepsis, and mortality. (IHI, 2019)
Pressure sores have a variety of treatments. Advanced sores are slow to heal, so early treatment is important (MedLine Plus, 2018).
Pressure ulcers are often associated with nursing homes and long-term skilled care facilities, but some 60,000 deaths occur each year from complications due to hospital-acquired pressure ulcers (Sullivan & Schoelles, 2013).
Unlike many other medical errors, however, the incidence of pressure ulcers has done more rising than falling. Data from 1995 to 2008 showed a climb by perhaps by as much as 80% in the incidence of pressure ulcers (Sullivan & Schoelles, 2013). And, while they increased again from 2014 to 2015, they decreased somewhat from 2015 to 2016 and 2017 (AHRQ, 2019e).
Preventing Pressure Ulcers
An Area Where Pressure Ulcers are Common
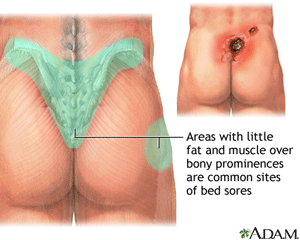
Source: MedLine Plus.
“Preventing pressure ulcers entails to two major steps: first, identifying patients at risk; and second, reliably implementing prevention strategies for all patients who are identified as being at risk” (IHI, 2019). The IHI How-to Guide: Prevent Pressure Ulcers (IHI, 2011) (recommended through the AHRQ pressure ulcer prevention site) is available from the IHI website.
Basic preventions for pressure ulcers involve:
- Keeping skin clean and dry
- Changing position every two hours
- Using pillows and products that relieve pressure (MedLine Plus, 2018)
In healthcare settings, key changes recommended by the IHI How-to Guide include:
- Inspect skin daily
- Manage moisture on skin
- Conduct a pressure ulcer admission assessment for all patients
- Minimize pressure
- Optimize nutrition and hydration
- Reassess risk for all patients daily (IHI, 2019, 2011)
Positioning in Bed to Prevent Pressure Sores [1:17]
https://facingdisability.com/expert-topics/whats-the-most-important-thing-to-do-to-prevent-pressure-sores/mary-zeigler-ms
Mary Ziegler, the nurse who appeared in the previous video, is a clinical nurse specialist at the Rehabilitation Institute of Chicago (facingdisability.com. 2019), makes this her final word:
Adhere to the pressure relieving schedule like a religion!
In their large literature review, researchers Sullivan and Schoelles found evidence of that several key elements improved care and reduced pressure ulcer rates. These prevention measures include simplification and standardization of pressure ulcer–specific interventions and documentation, involvement of multidisciplinary teams and leadership, use of designated skin champions, ongoing staff education, and sustained audit and feedback (Sullivan & Schoelles, 2013).
Recent studies suggest that facilities implementing these kinds of system-wide improvements are reducing the incidence of pressure-ulcers in patients (Ackroyd-Stolarz, 2018; Englebright et al., 2018).
Documentation Errors
Central to many types of medical errors are issues with documentation. While illegible physician handwriting is often regarded as a humorous cliché, it can have ramifications that are anything but humorous. Charting errors or omissions can have serious consequences.
Electronic medical records (EMRs) and more comprehensive systems known as electronic health records (EHRs) have grown exponentially in the last two decades, bolstered by several game-changing pieces of legislation. For all their efficiencies, electronic records are still subject to documentation and informational errors.
High-risk copy-and-paste errors, which are defined as mistakes with high potential risk for patient harm, fraud, or tort claim, have been reported in 10% of patient EMRs. Such errors can result in inaccuracies that can carry forward throughout the patient’s record (Hirschtick, 2012).
Cho and colleagues, in a study at a 950-bed teaching hospital, found that more than 50% of medication orders entered through a computerized physician order/entry system had at least one error. Further, documentation errors occurred in 205 (82.7%) of 248 correctly performed administrations. When tracking incorrectly entered prescriptions, 93% of the errors were intercepted by nurses, but two-thirds of them were recorded as prescribed rather than administered (Cho et al., 2014).
Another study found a small but concerning error rate of as much as 0.05% in patient-note mismatches, where a clinical note pertaining to one patient was included in the electronic record of another patient (Wilcox et al., 2011).
Preventing Documentation Errors
Accurate documentation—written or electronic—is one of the most fundamental components in the medical record and is threaded through all quality indicators. For example, NQF has specific mentions of documentation as part of a number of its measures including:
- 0045. Communication with the physician or other clinician managing on-going care post fracture for men and women aged 50 years and older
- 0092. Emergency Medicine: Aspirin at Arrival for Acute Myocardial Infarction
- 0419e. Documentation of Current Medications in the Medical Record (NQF, 2019)
Despite important improvements brought by electronic medical records, such as no longer needing to decipher illegible handwriting and having faster access, there are challenges—both technological and human—to be identified and resolved. And, not every aspect of care has the same issues; what is needed in the emergency department may differ from what is needed elsewhere in a hospital (Luthra, 2016; PSNet, 2019b).
A 2015 article in the Journal of the American Health Information Management Association identified the top four documentation errors (“disasters”) as:
- Mixed messages from a physician vis á vis misunderstood dictation or illegible handwriting
- Misuse of copy-and-paste or copy forward functions in the electronic health record (EHR)
- Incomplete or missing documentation
- Misplaced documentation (Butler, 2015)
After identification of the most frequent errors, the next step is putting procedures in place to prevent them. In response to the need for increased patient safety, changes to pay-for-performance programs, and closer monitoring by outside agencies, clinical documentation improvement (CDI) programs are growing. Making changes and training healthcare professionals in new habits is challenging but critical (Butler, 2015).
For all the improvements brought by EHR systems and their required use, there is no getting around “Garbage in, garbage out.” Electronic systems will not fix problems caused by poor training, inattention to detail, typos, mistakes using copy-and-paste, and other human errors. Continued improvements in technology and training of those who use it are essential (PSNet, 2019b).
A 78-year-old man with hypertension and diabetes presented to an emergency department (ED) with new-onset chest pain. The ED physician reviewed the patient's electronic medical record (EMR) and noted “PE” listed under past medical history, which raised his suspicion for the possibility of a new pulmonary embolus (PE). After initial testing excluded a cardiac etiology, a computed tomography (CT) scan of the chest was ordered to rule out a PE. When the physician approached the patient to explain why he was ordering the diagnostic test, the patient denied ever having a PE or being treated with blood thinners.
Puzzled by the conflicting reports, the ED physician returned to the EMR and noted that this mistaken history of PE dated back several years. It even appeared in the “problem list” section of his EMR. Investigating further back, the ED physician discovered that the letters “PE” were first noted nearly a decade earlier where it was clearly intended to reflect a “physical examination” rather than a “pulmonary embolus.” A physician likely copied and mistakenly pasted “PE” under “past medical history," after which this history of pulmonary embolism was carried forward time and time again.
The patient, who was ultimately discharged from the ED, never suffered any harm from the documentation error. The EMR was updated to say “This patient never had a pulmonary embolism.”
Source: Hirschtick, 2012.
