There is no cure for Parkinson’s Disease, so therapies are designed to alleviate symptoms and delay the progressive effects of the disease, and its treatments, for as long as possible. With that goal in mind, best treatment may vary for each patient and should be re-evaluated as symptoms change. Current treatments are effective at managing the early motor symptoms of the disease, though the patient should be encouraged to continue as many daily activities as possible and consider physical therapy to maintain motor skills and range of motion.
The antiparkinson drugs that are often employed to alleviate motor symptoms in early stages are levodopa (usually combined with a dopa decarboxylase inhibitor (DDCI) or COMT inhibitor), dopamine agonists, and MAO-B inhibitors. Initial drug treatment may start with MAO-B inhibitors and dopamine agonists to see if symptoms are sufficiently controlled. Levodopa (L-DOPA) plus a DOPA decarboxylase inhibitor (DDCI, such as carbidopa) are used sparingly at first to delay as long as possible the side effects resulting from cumulative exposure of systemic dopaminergic function.
As the disease progresses and dopaminergic neurons continue to be lost in the substantia nigra, these drugs eventually become ineffective for treating the motor symptoms, and at the same time cause a complication known as dyskinesia, marked by involuntary writhing movements. As medication becomes less effective, “off” periods may occur when the medication has worn off and movement is again difficult until a new dose is given. Medications to treat non-movement-related symptoms of PD, such as sleep disturbances and emotional problems, are also considered as needed (Brunton et al., 2011).
Diet and some forms of rehabilitation have shown some effectiveness at alleviating symptoms. Surgery and deep brain stimulation have been used to reduce motor symptoms when drugs are no longer effective or have too many side effects. Palliative care is given to enhance the quality of life in the final stages of disease.
Research is active to find better animal models of the disease and to advance new techniques using real-time, non-invasive imaging of the brain, stem cell, and gene therapies. New possibilities are being developed to reprogram cells, derived from the patient to avoid immune rejection, for tissue or functional replacement of some deficit.
The Dopamine Pathway
Dopamine is a neurotransmitter, conveying messages from one nerve cell to another. The nigrostriatal pathway in the midbrain, associated with motor control, is an information network for dopamine signaling.
Dopamine Pathways in the Brain
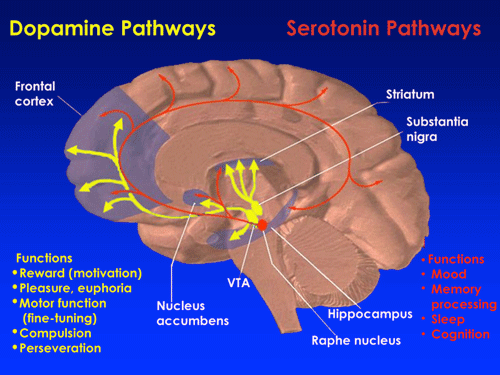
The nigrostriatal pathway makes use of dopamine signaling from the substantia nigra to the striatum. Source: NIDA, 2013.
Dopamine is secreted from membrane storage vesicles in the presynaptic neuron and binds to and activates dopamine receptors on the postsynaptic neuron to mediate its physiologic effects (Brunton et al., 2011). After dopamine has signaled from one neuron to another at the synapse, it is removed via re-uptake back into the presynaptic cell by either the high-affinity dopamine transporter (DAT) or the low-affinity plasma membrane monoamine transporter (PMAT). Once back inside the cytosol, it is eventually repackaged into vesicles for new signaling.
Alternatively, dopamine is directly broken down into inactive metabolites by two enzymes, monoamine oxidase (MAO) and catechol-O-methyl transferase (COMT). In most areas of the brain, including the striatum and basal ganglia, dopamine is inactivated by re-uptake via the DAT, then enzymatic breakdown by MAO. MAO inhibitor drugs help to retain dopamine for a longer time, allowing more synaptic signaling where dopamine is helping to alleviate PD symptoms.
Insufficient dopamine biosynthesis due to loss of the substantia nigra dopaminergic neurons can cause Parkinson’s disease and loss of ability to execute smooth, controlled movements; thus, one net effect of dopamine depletion is to produce hypokinesia, an overall reduction in motor output. Drugs that are used to treat PD may, however, produce excessive dopamine activity, allowing motor systems to be activated excessively and producing dyskinesias (jerky motions, spasms, tics).
Levodopa is the precursor to dopamine used for various forms of Parkinson’s disease and dopa-responsive dystonia (twisting muscle contractions and postures). Dopamine is unable to cross the blood-brain barrier (BBB) directly. It is usually co-administered with an inhibitor of peripheral decarboxylation enzymes such as carbidopa or benserazide, to allow more levodopa to survive long enough to cross the BBB. Inhibitors (entacapone or tolcapone) of COMT—an alternative metabolic route for dopamine—are also used.
The long-term use of levodopa in Parkinson’s disease has been linked to dopamine dysregulation syndrome—hence the attempt to delay and minimize use for as long as possible.
Medications
The mainstay of the treatment of Parkinson’s disease is pharmacologic replacement of dopamine, most commonly accomplished with the precursor of dopamine, levodopa—usually combined with a DDCI or COMT inhibitor (see below), dopamine agonists, and MAO-B inhibitors. Initial drug treatment may start with MAO-B inhibitors and dopamine agonists to see if symptoms are sufficiently controlled (Brunton et al., 2011).
Levodopa (L-DOPA)
The most widely used drug for PD is levodopa (L-DOPA). Nerve cells can use levodopa to make dopamine and replenish the brain’s dwindling supply. L-DOPA is converted to dopamine in the dopaminergic neurons by the enzyme dopa decarboxylase, temporarily diminishing motor symptoms of PD. Although levodopa helps in at least three-quarters of parkinsonian cases, not all symptoms respond equally to the drug. Bradykinesia and rigidity respond best, while tremor may be only marginally reduced. Problems with balance and other symptoms may not be alleviated at all.
Only about 10% of a dose of L-DOPA actually crosses into the brain while the rest is susceptible to conversion to dopamine in the periphery, leading to side effects such as nausea, dyskinesias, and joint stiffness. For that reason, peripheral dopa decarboxylase inhibitors (DDCI), such as carbidopa and benserazide, are given in combination with levodopa to reduce peripheral conversion that would otherwise devour most of the dose given. This also maximizes bioavailabilty for the brain, decreases side effects, and allows a lower dose of levodopa to be used.
Controlled-release versions of levodopa in the form of intravenous and intestinal infusions spread out the medication but have not shown a better control of motor symptoms or complications than standard forms.
Tolcapone inhibits the catechol-O-methyltransferase (COMT) enzyme, which degrades dopamine, prolonging the effects of levodopa, though its usefulness is limited by possible liver toxicity. A similar drug, entacapone, has not shown the liver complications and is used alone or in combination with carbidopa plus levodopa (Sinemet).
After prolonged therapy with levodopa, a person with PD can alternate from phases with good response to medication and few symptoms (the “on” state), to phases with no response to medication and significant motor symptoms (the “off” state); therefore, levodopa doses are kept as low as possible after using alternatives such as dopamine agonists and MAO-B inhibitors. Most people with PD will eventually require levodopa and hence later develop motor side effects such as involuntary movements (dyskinesia), painful leg cramps (dystonia) and a shortened response to each dose (motor fluctuations) (Huot et al., 2013).
Carbidopa Plus Levodopa
Carbidopa (Lodosyn) is used with levodopa (Sinemet, Parcopa, Atamet) to prevent the peripheral conversion of levodopa to dopamine before it can reach the brain and take effect. It also reduces the side effects of peripheral dopamine. Carbidopa is only effective if it is taken with levodopa; it has no effect if used alone. Carbidopa inhibits dopa decarboxylase (DDC), an enzyme important to converting L-DOPA to dopamine. Recall that dopamine itself cannot cross the blood brain barrier. DDC is found in the periphery and in the brain, but carbidopa does not cross the BBB so it does not inhibit conversion of L-DOPA to dopamine there.
Entacapone (Comtan), a COMT inhibitor, is used in combination with Sinemet for times when levodopa stops working at the end of a dose—the “off” times. This drug prevents breakdown of dopamine by inhibiting the enzyme catechol-O-methyltransferase (COMT).
Dopamine Agonists
Dopamine agonists are molecules that bind to the postsynaptic dopamine receptors and mimic the role of dopamine in the brain, causing a response similar to dopamine itself. These were initially used to help alleviate the “off” state of late PD when the benefits of levodopa doses were wearing off. Now they are used as an early alternative to levodopa so that later complications and dyskinesias are postponed for as long as possible. Dopamine agonists include
- Bromocriptine (Parlodel, Cycloset)
- Pramipexole (Mirapex)
- Ropinirole (Requip)
- Piribedil (Pronoran, Trivastal Retard, Trastal, Trivastan)
- Cabergoline (Dostinex, Cabaser)
- Apomorphine (Apokyn, Ixense, Spontane, Uprima)
- Lisuride (Dopergin, Proclacam, Revanil)
Ropinirole (Requip) and Pramipexole (Mirapex) are nonergot dopamine agonists also used for restless legs syndrome.
Dopamine agonists produce significant, though usually mild, side effects including drowsiness, hallucinations, insomnia, nausea, and constipation. If side effects appear even at a minimal effective dose, another of this class of drugs can be tested as an alternative. These drugs are less able to control motor symptoms than levodopa, but they are usually sufficient in the earliest stages of the disease.
Agonists have been related to impulse control disorders (such as compulsive sexual activity and eating, and pathological gambling and shopping) more strongly than levodopa.
Apomorphine may be used to reduce “off” periods and dyskinesia in late PD, though it requires injections or continuous subcutaneous infusions and may cause confusion and hallucinations. Apomorphine treatment obviously requires close attention from caregivers. Two other dopamine agonists are available as skin patches (lisuride and rotigotine) and have benefit in early stages and for the “off” state in advanced stages of PD.
MAO-B Inhibitors
Selegiline (Eldepryl, Deprenyl, or Selgene) and rasagiline (Azilect) are MAO-B inhibitors that increase the level of dopamine in basal ganglia synapses by blocking its metabolism. They inhibit the monoamine oxidase-B (MAO-B) enzyme responsible for breaking down dopamine secreted by the dopaminergic neurons. Like dopamine agonists, MAO-B inhibitors alone can improve motor symptoms and delay the need for levodopa use in early disease but they are less effective than levodopa. In the advanced disease, they can be used to reduce fluctuations between “on” and “off” periods. None of these treatments slow the progression of the disease.
Other Drugs
Amantadine (Symmetrel) is a weak antagonist of NMDA-type glutamate receptors, increases dopamine release, and blocks dopamine re-uptake in the synapse. It can be taken with Sinemet to treat motor response fluctuations in advanced disease.
Anticholinergics that block the neurotransmitter acetylcholine in the central and peripheral nervous system may be useful as treatment of motor symptoms by essentially anesthetizing the muscle/ nerve connections to reduce unwanted motor symptoms and rigidity.
Several drugs have been used to treat other symptoms common to PD patients, such as the use of clozapine (Clozaril, FazaClo) for psychosis, cholinesterase inhibitors for dementia, and modafinil for daytime sleepiness. Some studies have implied that regular users of nonsteroidal anti-inflammatory drugs (NSAIDs, apart from acetaminophen and aspirin), have a lower risk of ever developing PD.
Other medications may also include:
- Memantine (Namenda), rivastigmine (Exeleon), galantamine (Razadyne) for cognitive difficulties; these are NMDA-receptor antagonists or acetylcholinesterase inhibitors
- Antidepressants for mood disorders
- Gabapentin (Neurontin, Gralise, Fanatrex) to treat certain types of seizures or restless legs syndrome
- Duloxetine (Cymbalta) treats depression, anxiety, peripheral neuropathy (nerve pain), fibromyalgia (muscle pain and stiffness), or chronic pain related to muscles and bones. This is a selective serotonin and norepinephrine reuptake inhibitor (SSNRI).
- Fludrocortisone, midodrine, botox, sidenafil for autonomic dysfunction
- Armodafinil (Nuvigil), clonazepam (Klonopin), zolpidem (Ambien) for sleep disorders and daytime wakefulness
Surgical Intervention
Deep Brain Stimulation
Surgery may be an option for some patients with Parkinson’s disease. These surgeries do not cure Parkinson’s but may help ease symptoms. Deep brain stimulation (DBS), using high-frequency stimulating electrodes, was approved by the FDA in 1997 and has been a promising avenue for treatment of movement disorders. DBS is recommended for people who have PD and suffer from motor fluctuations and tremor inadequately controlled by medication or for those who are intolerant of medication, as long as they do not have severe neuropsychiatric problems (Okun, 2012).
Stimulation of the ventral intermediate nucleus of the thalamus (VIM) can show marked reduction in the tremors associated with PD. Stimulation of the subthalamic nucleus (STN) or the internal segment of the globus pallidus (GPi) can greatly reduce tremor, rigidity, bradykinesia, and difficulty with walking and controlled movement.
The results of stimulation for PD depends on which region receives DBS. VIM stimulation primarily reduces limb tremor. Targeting the GPi appears to reduce all of the major motor problems with PD, including those dyskinesias that arise after extended use of levodopa. It does not appear to alleviate other issues with drug side effects such as psychosis or cognitive impairment. Subthalamic nucleus DBS also appears to reduce most of the motor symptoms and, if performed on both sides of the brain, may allow reduction in dopaminergic medications and their associated side effects.
While the effects of DBS are not more effective than a dose of levodopa, it does seem to reduce the time spent in the “off” state when the medication has worn off and symptoms reappear, and it does allow a reduction in levodopa so that those side effects are pushed further into the future. The benefits of DBS are typically maintained for at least four years before other complications arise.
The best candidates for DBS are those who are exhibiting negative effects from levodopa exposure yet had been having motor benefit from the oral drugs. However, memory, disorientation, and other cognitive problems may be increased by DBS and may be sufficient reasons for not using it if these are already a clinical concern. There are still major questions as to the mechanisms of action for DBS, and research to improve it are ongoing.
Patient Undergoing DBS Surgery
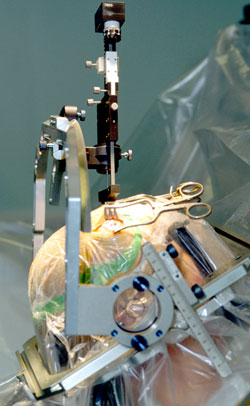
Placement of an electrode into the brain. The head is stabilized in a frame for stereotactic surgery. Source: Wikimedia Commons.
Clinically, DBS is a two-stage procedure involving in the first stage a stereotactic frame, with the patient under sedation yet awake, for a 30-minute, three-dimensional MRI to locate the coordinates of the deep brain target. After determining the target, while still in the operating room a path for the very fine metal electrodes is planned that will reach the target. The DBS electrode is placed to the target, and electrical impulses are sent to see which placement gives the best reduction in tremors, while monitoring for other unwanted side effects in speech or numbness.
Once an effective place is found, the electrode is left in and clipped into place on the skull, and the exterior wound is closed. A second operation is performed under general anesthesia to place a small battery pouch containing the stimulator pulse generator under the collarbone. From there, a wire is passed under the skin up the neck to behind the ear, where it re-emerges and is attached to the stimulator wire into the brain.
After observation for several weeks, the unit will be turned on and tested further. Depending on the targeted region of the brain, a neurologist will be involved with the delicate electrode placement, and one or both sides of the brain may be targeted, in similar but separate operations.
The expected result of DBS is to relieve some or most of the motor dysfunction. This can often result in an improvement of the “off” state, when medication has had less benefit and motion is again stiff or slow. The “on” state, when medication is effective, is not further improved by DBS. Followup exams can allow externally programmed modifications of the stimulation frequency or intensity to optimize the benefit for each particular patient and the brain region targeted.
Pallidotomy
Some people with Parkinson’s disease benefit from other types of neurosurgical procedures such as pallidotomy. Pallidotomy is a procedure whereby a tiny electrical probe is placed in the globus pallidus (GPi, one of the basal ganglia of the brain), which is then heated to 80°C for 60 seconds to ablate a small area of brain cells. Pallidotomy is an alternative to deep brain stimulation for the treatment of a condition known as levodopa-induced dyskinesia, the involuntary movements that can become a problem in people with PD after long-term treatment with levodopa. It can be an alternative to DBS for treating difficult cases of essential tremor.
Animal Models
It is thought that no other species than humans naturally develop PD, although animal models have been developed for research. In the early 1980s the appearance of parkinsonian symptoms in a group of drug addicts who consumed a contaminated batch of the synthetic opiate MPPP led to the discovery of the chemical MPTP as an agent that causes a parkinsonian syndrome in nonhuman primates as well as in humans. Other toxin-based models of PD employ the insecticide rotenone, the herbicide paraquat, or the fungicide maneb. Models based on toxins are most commonly used in primates. Transgenic rodent models that replicate various aspects of PD have also been developed for understanding or testing treatments for specific components of PD symptoms.
Gene and Stem Cell Therapies
There is consensus that new treatments move from treating symptoms to modifying the disease pathology, while also aiming to reduce the nonmotor disease symptoms such as loss of balance, autonomic dysfunction, and cognitive impairment that come with time and diminish quality of life.
Gene therapy involves the use of DNA in a noninfectious virus to shuttle a gene into a part of the brain. The gene used is meant to increase the production of an enzyme that helps to manage PD symptoms or protects the brain from further damage. In 2010 there were four clinical trials using gene therapy in PD and none of them have had serious adverse effects, though the clinical benefit is also undecided.
Clinical studies have focused on the therapeutic potential of neurotrophic factors, including GDNF and neurturin, and enzymes that produce dopamine. One trial is using adenovirus-associated virus (AAV2) to deliver the gene for the enzyme that converts levodopa to dopamine, hoping to make this more abundant and thereby allowing a lower dose to be used. Another trial is using AAV2-mediated delivery of neurturin, a functional analog of glial cell-derived neurotrophic factor (GDNF), which aims to provide neuroprotective benefits in addition to symptomatic improvement. Neurturin provides neuroprotection and upregulation of dopamine function in a variety of rodent and nonhuman primate models. One of the human trials using gene transfer of glutamic acid decarboxylase (GAD) enzyme to improve GABA production in the subthalamic nucleus reported positive results for PD patients in 2011.
Since the 1980s, fetal porcine carotid body cells or immature retinal tissues have been used in cell transplants, in which dissociated cells are injected into the substantia nigra hoping that they incorporate themselves into the brain and replace the dopamine-producing cells that have been lost. Though mesencephalic dopamine-producing cell transplants were initially positive, further trials did not show benefit beyond other types of current therapy. In some cases the new cells were secreting more dopamine than was necessary, leading to the dystonias common in advanced PD.
Stem cell transplants are a recent research target, because stem cells are easy to grow and manipulate, and when transplanted into the brains of rodents and monkeys they have been found to survive and reduce abnormalities. New possibilities are recently available to re-program cells by using induced pluripotent stem cells, derived from the patient to avoid immune rejection, for tissue or functional replacement cells.
Several molecules have been proposed as potential treatments for neuroprotection in PD patients. However, none of them have been convincingly shown to reduce degeneration. Currently promising molecules include:
- Anti-apoptotics (omigapil, CEP-1347)
- Antiglutamatergics
- Monoamine oxidase inhibitors (selegiline, rasagiline)
- Promitochondrials (coenzyme Q10, creatine)
- Calcium channel blockers (isradipine)
- Growth factors (GDNF)
Oral supplementation with co-enzyme Q10, a mitochondrial complex I electron-accepting antioxidant, reduced dopaminergic neuron loss in MPTP-toxin treated mice. A vaccine that primes the human immune system to destroy alpha-synuclein, the main component of Lewy bodies, has entered clinical trials in humans. Whether this will help PD patients remains to be seen. Stem cell transplant and other clinical trials are currently ongoing in the United States. For more, click here.
Lifestyle Recommendations
[This section taken primarily from NIH, 2012.]
Individuals with Parkinson’s disease may benefit from physical, occupational, and speech therapy. Several major organizations promote research and improving quality of life for those with the disease and for their families.
Lifestyle changes that you can recommend for the person with Parkinson’s disease:
- Good general nutrition and health. Changes in food or drink are needed if there are swallowing problems.
- Exercising every day, but adjusting the activity level to meet changing energy levels
- Regular rest periods and avoiding stress
- Physical therapy, speech therapy, and occupational therapy. A physiotherapist can suggest appropriate stretches and exercises.
- Keeping informed on specific medical conditions.
- Joining a support group.
- Talking to your physician about when and what type of medication you can take.
- Railings or banisters placed in commonly used areas of the house. Other changes may be needed around the home to prevent falls and make the bathroom safe.
- Assistive devices, such as special eating utensils, wheelchairs, bed lifts, shower chairs, walkers, and wall bars
- Social workers or other counseling services to help you cope with the disorder and get assistance (eg, Meals-on-Wheels)
- Continue doing what makes you happy.
A wide variety of complementary and supportive therapies may be used for Parkinson’s disease. Among these therapies are standard physical, occupational, and speech therapies, which help with gait and voice disorders, tremors and rigidity, and decline in mental functions. Other supportive therapies include diet and exercise.
Diet
At this time there are no specific vitamins, minerals, or other nutrients that have any proven therapeutic value in Parkinson’s disease. Some early reports have suggested that dietary supplements might protect against PD. Also, a preliminary clinical study of a supplement called coenzyme Q10 suggested that large doses of this substance might slow disease progression in people with early-stage Parkinson’s. This supplement is now being tested in a large clinical trial.
Other studies are being conducted to find out if caffeine, antioxidants, nicotine, and other dietary factors may help prevent or treat the disease. While there is currently no proof that any specific dietary factor is beneficial, a normal healthy diet can promote overall well-being for people with Parkinson’s disease, just as it would for anyone else. Eating a fiber-rich diet and drinking plenty of fluids can help alleviate constipation. A high protein meal, however, may limit levodopa’s effectiveness because for a time afterwards less levodopa passes through the blood-brain barrier while competing with other amino acids. Therefore, when levodopa is introduced, excessive protein consumption is discouraged and a well-balanced Mediterranean diet is recommended. In advanced stages, additional intake of low-protein products such as bread or pasta is recommended for similar reasons. To minimize interaction with proteins, levodopa should be taken 30 minutes before meals.
Exercise
Exercise can help people with Parkinson’s disease improve their mobility and flexibility. Some doctors prescribe physical therapy or muscle-strengthening exercises to tone muscles and to put under-used and rigid muscles through a full range of motion. Exercises will not stop disease progression, but they may improve body strength so that the person is less disabled. Exercises also improve balance, helping people minimize gait problems, and can strengthen certain muscles so that people can speak and swallow better.
Exercise can also improve the emotional well-being of people with Parkinson’s disease, and it may improve the brain’s dopamine synthesis or increase brain levels of beneficial compounds called neurotrophic factors. Although structured exercise programs help many patients, more general physical activity, such as walking, gardening, swimming, calisthenics, and using exercise machines is beneficial. People with Parkinson’s disease should always check with their doctors before beginning a new exercise program.
In terms of improving flexibility and range of motion for patients experiencing rigidity, generalized relaxation techniques such as gentle rocking have been found to decrease excessive muscle tension. Other effective techniques to promote relaxation include slow rotational movements of the extremities and trunk, rhythmic initiation, diaphragmatic breathing, and meditation.
Strengthening exercises have shown improvements in strength and motor function for patients with primary muscular weakness and weakness related to inactivity with mild to moderate PD. However, reports show a significant interaction between strength and the time the medications was taken, so it is recommended that patients should perform exercises 45 minutes to 1 hour after medications, when the patients are at their best. Also, due to the forward flexed posture and respiratory dysfunctions in advanced Parkinson’s disease, deep diaphragmatic breathing exercises are beneficial in improving chest wall mobility and vital capacity.
A common treatment for speech disorders associated with Parkinson’s disease is the Lee Silverman voice treatment (LSVT). Occupational therapy (OT) aims to promote health and quality of life by helping people with the disease to participate in as many of their daily living activities as possible.
Other Therapies
Other complementary therapies include massage therapy, yoga, tai chi, hypnosis, acupuncture, and the Alexander technique, which improves posture and muscle activity. There have been limited studies suggesting mild benefits from some of these therapies, but they do not slow Parkinson’s disease. However, this remains an active area of investigation.
