Modes and mechanisms of transmission of pathogens organisms in the healthcare setting and strategies for prevention and control.
It is becoming increasingly clear that transmission of infections in healthcare settings is largely preventable through the use of evidence-based IC guidelines. The concept of the chain of infection provides the basis for understanding the transmission of pathogens as well as identifying practices and procedures to prevent healthcare-associated infections.
For the purposes of NHSN surveillance in the acute care setting, a healthcare-associated infection (HAI) is a localized or systemic condition resulting from an adverse reaction to the presence of an infectious agent(s) or its toxin(s) that was not present on admission to the acute care facility (CDC, 2014).
Antibiotic-resistant organisms have changed the infection control landscape. Methicillin-resistant S. aureus (MRSA), C. difficile, and vancomycin-resistant enterococcus (VRE), among others, have become serious problems in healthcare facilities over the past two decades. The MRSA organism alone is responsible for more than 94,000 invasive infections and almost 19,000 deaths each year in the United States (Klevens et al., 2007).
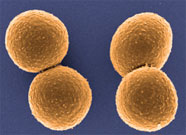
This colored electron micrograph shows isolated S. aureus bacteria that are resistant to many forms of antibiotics. Source: NIAID.
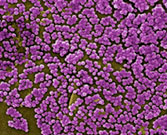
Scanning electron micrograph depicting numerous clumps of MRSA bacteria. Source: CDC.
Bad Bugs, No Drugs
In 2004 the Infectious Diseases Society of America (IDSA) raised the alarm about the dramatic increase in drug-resistant bacteria and the diminishing supply of new antibiotics in its landmark Bad Bugs, No Drugs report.
The Society has pursued multiple approaches, including legislation, to strengthen federal leadership, public health efforts, research, data collection, surveillance, appropriate use strategies, and efforts to fix the anemic drug pipeline.
In 2010 IDSA launched its 10 by 20 Initiative, calling for political leaders, regulators, manufacturers, etc., to work together to create an infrastructure capable of developing 10 new systemic antibiotics by 2020. Only one 10 x 20 antibiotic has been approved to date.
Source: IDSA, 2014.
Muto and colleagues (2003), in a ground-breaking document for the Society for Healthcare Epidemiology of America, published the SHEA guideline for preventing nosocomial transmission of multidrug-resistant strains of Staphylococcus aureus and Enterococcus.
While most types of HAIs are declining, one, caused by the germ Clostridium difficile, remains at historically high levels. Clostridium difficile causes diarrhea linked to 14,000 American deaths each year (CDC, 2013). Those most at risk are people, especially older adults, who take antibiotics and also get medical care. Clostridium difficile has also become more virulent, and hospital-associated outbreaks are causing increased deaths. In the general population, C. difficile is present in about 5% of the population. The need to control outbreaks of C. difficile has focused new attention in the area of environmental cleaning. Because C. difficile causes watery diarrhea it can spread easily and rapidly in the healthcare setting, passing from person to person via clothing, equipment, and dirty hands.
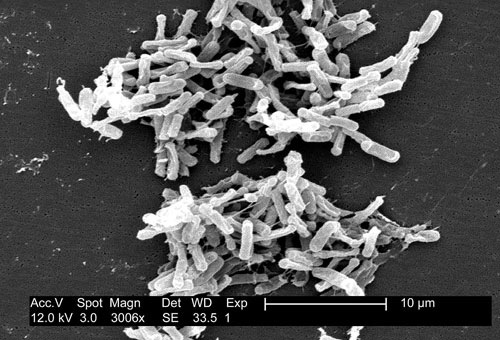
Scanning electron micrograph of Clostridium difficile bacteria from a stool sample. Source: CDC/Lois S. Wiggs (PHIL #6260), 2004.
Vancomycin-resistant enterococcus (VRE) is another antibiotic-resistant organism that has been associated with increased mortality and length of hospital stay. Many studies have shown that VRE can be readily found on cabinets, bed rails, equipment, and bedside tables and it is easily transmitted on the hands and gloves of healthcare workers. Vancomycin-resistant enterococcus is also easily transmitted on equipment such as blood pressure cuffs, stethoscopes, pulse oximeters, IV poles, telephones, and infusion pumps. Aggressive environmental cleaning, screening of incoming patients for VRE and MRSA, isolation, and stringent barrier precautions have led to remarkable success in controlling and eliminating these organisms in hospitals in Denmark, Finland, and The Netherlands (Muto et al., 2003).
An illustration of the influenza virus micro-organism. Source: Illustration provided by 3DScience.com.
Even before the H1N1 outbreak of 2009, influenza had long been an area of focus for infection prevention. Although flu pandemics have occurred periodically for centuries, causing hundreds of thousands of deaths, we now have the ability to identify an influenza epidemic as it is emerging. We also have the public health capability to take action (vaccines, surveillance, education) to minimize the impact of a flu outbreak. Addressing and controlling these emerging threats has become a priority for healthcare organizations. (See ATrain’s course, Influenza: Fighting Complacency, which is updated every year.)
The Chain of Infection
The Chain of Infection
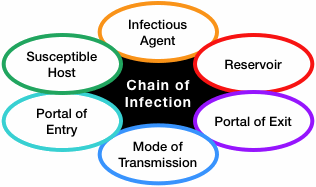
We have all seen infections spread through a family, classroom, or office; this situation can be described using a concept called the chain of infection (see figure below). It is a process that begins when (1) an infectious agent or pathogen (2) leaves its reservoir, source, or host through (3) a portal of exit, (4) is conveyed by some mode of transmission, (5) enters the host through an appropriate portal of entry, and (6) infects a susceptible host. The now-infected susceptible host becomes a new reservoir and the whole process starts over.
The concept of a chain of infection is essential to our understanding of why we do what we do to prevent infection. If any one link of the chain of infection can be broken, the spread of infection can be prevented.
Infectious Agents (Pathogens)
Infectious agents are the microorganisms or “germs”—bacteria, viruses, fungi, and protozoa—that can cause disease or illness in their hosts. Some microorganisms are pathogens, a word derived from the Greek, meaning “that which produces suffering.” Although microorganisms are common in the environment, most are not harmful to people.
Pathogens vary in infectivity and virulence, and to cause disease an infectious dose (a sufficient number of organisms) is required. Creating an environment with no pathogens is not a realistic goal outside of highly specialized laboratories.
Bacteria
Bacteria are single-celled organisms, the vast majority of which are harmless or even beneficial. Our bodies contain bacteria, called normal flora, that protect us from infection by providing competition to pathogens. Normal flora usually do not cause disease unless balance is disturbed or the bacteria get into a part of the body that cannot tolerate them. Antibiotics are effective against many bacterial infections although, as already noted, the overuse or misuse of antibiotics has produced strains of bacteria that are resistant to them.
Pathogenic bacteria contribute to a number of globally prevalent diseases, including pneumonia, tuberculosis, and bacterial meningitis. Pathogenic bacteria include group A and group B streptococcus, Haemophilus influenzae, Staphylococcus aureus, including MRSA, Clostridium difficile, Neisseria meningitidis, and Streptococcus pneumoniae.
A coccus is a bacterium with a spherical shape. Chains of cocci indicate streptococcus, while clusters indicate staphylococcus. Source: Illustration provided by 3DScience.com.

Bacillus can refer to any rod-shaped bacterium, or can be more specific to Bacillus, which is a gram-positive and rod-shaped genus. Source: Illustration provided by 3DScience.com.
Viruses
Viruses are true parasites in that they can only reproduce inside the host cell. More than five thousand types of viruses have been described since the first was discovered in 1899. Viruses are about a hundred times smaller than bacteria and, like bacteria, not all viruses cause disease.
Viruses spread in many different ways—by direct or indirect contact (soiled hands or articles), by droplets from coughing and sneezing, by contact with blood, sexual contact, by fecal contamination, by contaminated food and water, or via certain insects. Examples of diseases caused by viruses include influenza, chickenpox, West Nile fever, and HIV.
Antibiotics are not effective against viruses. Vaccines, however, have been successful in eliminating or controlling some viral disease—including smallpox, polio, measles, mumps, and rubella—that have killed millions of people throughout the world. Anti-viral medications for some illnesses have varying degrees of effectiveness.
This image of the West Nile Virus shows the characteristic rough and furrowed surface with no protein arms projecting from it, as so many viruses have. Source: Illustration provided by 3DScience.com.
HIV is a retrovirus, whose genetic content is stored in RNA, which is copied into the DNA of the host upon infection. Source: Illustration provided by 3DScience.com.
Fungi
Fungi are very common, but only a few cause diseases in humans. Some fungal infections are life-threatening in certain susceptible patients. Fungal infections can be superficial (limited to the surface of the skin and hair), cutaneous (extending into the epidermis, nails, and hair), or subcutaneus (extending into the dermis, subcutaneous tissues, muscle, and fascia). Fungal infections can also be systemic, often originating in the lungs and spreading to multiple organs. There are several classes of antifungal medications, although fungal and human cells are similar on the molecular level, so antifungal drugs can have mild to serious side effects. Athlete’s foot, yeast infections, and candidemia (yeast growing in the blood) are examples of diseases caused by fungi.
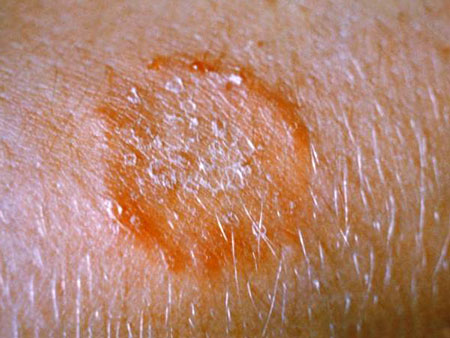
An example of a fungal infection called ringworm (no worm is involved). Source: CDC.
One fungus that survives well in air, dust, and moisture in healthcare facilities is Aspergillus spp., a ubiquitous, aerobic fungus that is present in soil, water, and decaying vegetation. Site renovation and construction can disturb Aspergillus—contaminated dust can produce bursts of airborne fungal spores, which have been associated with clusters of HAIs in immunocompromised patients. Absorbent building materials such as wallboard are an ideal growth medium for this organism if they become and remain wet. Patient-care items, devices, and equipment can become contaminated with Aspergillus spp. spores and serve as sources of infection (CDC, 2003).
Other opportunistic fungi that are occasionally linked with HAIs are members of the order Mucorales and molds such as Fusarium and Penicillium. Many of these fungi can proliferate in moist environments—for example, in water-damaged wood and building materials. Some fungi, such as Fusarium and Pseudoallescheria, can be airborne. As with aspergillosis, a major risk factor for disease caused by any of these pathogens is the host’s severe immunosuppression from either underlying disease or immunosuppressive therapy.
Protozoa
Protozoa are single- or multi-celled microorganisms that are larger than bacteria. They have traditionally been classified by their means of propulsion: flagella, amoeboid, sporozoan, or ciliate. They may be transmitted in soil, via water, by direct contact, or by an insect such as a mosquito.
Examples of diseases caused by protozoa include malaria and giardia. Malaria is a protozoan that lives in the blood of the host and is transmitted when an insect bites, ingests infected blood, and then transmits it by biting a new host.
Protozoa are less common than the other types of organisms in the United States and can be treated with specific medications.
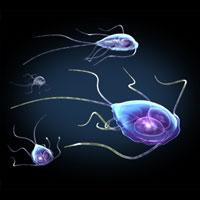
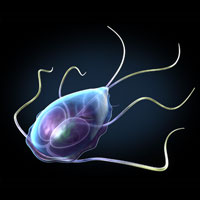
These images depict Giardia trophozoites in a variety of positions. Giardia stick closely to the lining of the small intestine in the hosts they infect and cause mild to severe diarrhea. Source: Illustration provided by 3DScience.com.
Parasites
Parasites are usually larger organisms that exploit a host by living on the skin, inside the gut, or in tissues. The life of a parasite is precarious because the host usually does everything it can to destroy the parasite. Parasites are dependent upon the host for survival and employ a number of strategies to move from host to host. They can be transmitted by direct contact, as with lice or scabies, or wait in the external environment until there is contact with the host (ticks, leeches).
Helminthes are a class of parasites that live inside the body and include roundworms, tapeworms, and flukes. They infect humans principally through ingestion of fertilized eggs or when the larvae penetrate the skin or mucous membranes.
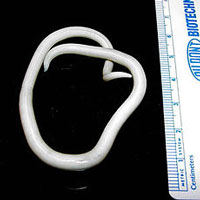
The parasitic roundworm Ascaris lumbricoides. As many as one-quarter of the world’s population is infected with Ascaris. Source: Wikipedia.

Pediculus humanus var capitis, also known as head louse. Source: Wikipedia.
Reservoirs
Reservoirs are the places where the germs live and grow. A general rule: If an area stays wet, it is probably a reservoir. The most common reservoirs in healthcare facilities are people, who may be sick or healthy.
Infectious agents transmitted during healthcare derive primarily from human sources. Human reservoirs include patients, healthcare personnel, and household members and other visitors. These source individuals may have active infections, may be in the asymptomatic or incubation period of an infectious disease, or may be transiently or chronically colonized with pathogenic microorganisms, particularly in the respiratory and gastrointestinal tracts (Siegel, 2007).
Surprisingly, reservoirs can be complex and difficult to identify. The CDC defines a reservoir as “one or more epidemiologically connected populations or environments in which the pathogen can be permanently maintained and from which infection is transmitted to the defined target population” (Haydon et al., 2014).
Although many emerging diseases of human, domestic animal, and wildlife populations are assumed to be maintained in reservoir hosts, these reservoirs may not be identified. Sometimes there is agreement as to where an infectious organism resides and a specific public health action is taken. For example, approximately one million pigs were slaughtered in Malaysia in 1999 to control Nipah virus; several million chickens were slaughtered in Hong Kong in 1998 and 2001 to prevent a projected pandemic of Influenza A virus; and several million cows were slaughtered in Britain to curtail the epidemic of bovine spongiform encephalopathy.
In other instances the identity of reservoirs is less clear; for example, the reservoirs that harbor emerging deadly viruses such as Ebola and Marburg are unknown. Incomplete understanding of reservoirs has hampered control of many diseases in Africa, such as Ebola, Buruli ulcer, and rabies (Haydon et al., 2014).
In humans, the reservoir and the susceptible host can be the same person and can cause disease if the person’s normal flora gets into the wrong part of the body. For example, oral flora getting into the lungs can cause aspiration pneumonia, skin flora contaminating an IV site can cause a site or bloodstream infection, and fecal flora contaminating the urinary tract can cause a urinary tract infection (UTI). This is why care must be taken to avoid carrying germs between different body sites of the same patient. The most effective prevention technique is to change gloves and do hand hygiene when going from a contaminated area to a cleaner area.
In healthcare facilities, activities aimed at eliminating reservoirs include:
- Treating people who are ill
- Handling and disposing of body fluids carefully
- Using sterile water in respiratory equipment
- Drying equipment before storing it
- Handling food safely and cooking meat thoroughly
- Monitoring soil and contaminated water in sensitive areas of the hospital and washing hands carefully after contact with either
- Vaccinating people
- Encouraging ill workers to stay home
Infection control practices should be followed in all settings where healthcare is delivered, including home care, although the relative risks of acquiring an infection may differ. In acute care, a patient’s risk for an HAI is related not only to the severity of illness and exposure to invasive interventions and devices but also to environmental risks, including exposure to other patients and inanimate reservoirs or pathogens. In home care, the rationale and strategy for use of precautions differ from those applied in hospitals. In most cases, the use of gowns, gloves, and masks in the care of homebound patients is recommended to protect the healthcare provider, not the patient.
Caregivers in the home may need to use respiratory protection only when caring for patients with pulmonary tuberculosis (Haydon et al., 2014).
Home care patients known to have a multidrug-resistant organism should be cared for using appropriate barriers. Although these organisms may not be a risk to providers, they may be transmitted to other home care patients through inanimate objects or hands. Reusable equipment such as stethoscopes and blood pressure cuffs should remain in the home. If practical, such patients should be seen as the last appointment of the day. If this is not possible, visits should be scheduled to avoid seeing at-risk patients—such as patients requiring wound care—after seeing a patient with multidrug-resistant organisms (Haydon et al., 2014).
Portals of Exit: How Pathogens Leave the Body
A pathogen leaves its reservoir or host through a portal of exit. The portal of exit usually corresponds to the site where the pathogen is located. For example, influenza viruses and M. tuberculosis exit from the respiratory tract, cholera exits its host in feces, and Sarcoptes scabiei in scabies skin lesions. Some bloodborne pathogens can exit by crossing the placenta from mother to fetus (eg, rubella, syphilis, toxoplasmosis), while others exit through cuts in the skin or needles (hepatitis B) or blood-sucking insects (malaria) (CDC, 2014a).
The portal of exit is the link of the chain over which we have the least control. Any break in the skin—such as natural anatomic openings or draining lesions—may be a portal of exit from a host. Any body fluid may carry infectious agents out of the body. Some bacteria (such as MRSA) live on the patient’s skin, so even dry skin contact may serve as the portal of exit.
Activities aimed at eliminating portals of exit in healthcare facilities include:
- Covering coughs and sneezes with a tissue
- Handling body fluids with gloves—followed by hand hygiene
- Keeping draining wounds covered with a dressing
- Staying home from work when you have wet lesions or weeping dermatitis
Means of Transmission
Did You Know. . .
Very few germs can fly—almost all have to be carried from one place to another. The means of transmission is the weakest link in the chain of infection, and it is the only link we can hope to eliminate entirely. Most infection control efforts are aimed at preventing the transport of germs from the reservoir to the susceptible host.
All types of precautions (standard, contact, droplet, and airborne) are designed to interrupt the means of transmission. These are reviewed later under “Prevention Strategies.” Direct and indirect contact are the most common means of transmission in the healthcare setting—from the hands of the caregivers and items that move patient to patient. Because it addresses the weakest link in the chain of transmission, hand hygiene is still the single most important procedure for preventing the spread of infection.
Items moving between patients should be cleaned after each use to avoid indirect contact transmission of pathogens.
|
Common Means of Transmission |
|
|---|---|
|
Type of contact |
Example |
|
Direct |
Person-to-person transmission of pathogens through touching, biting, kissing, or sexual intercourse |
|
Indirect |
Involves an intermediate person or item between the portal of exit and the portal of entry to the next person. Microorganisms may be carried by unwashed hands or soiled objects, called fomites. Any soiled object, such as blood-pressure cuffs, pens, bed rails, used tissues, soiled laundry, or doorknobs, may be a fomite. |
Indirect Transmission
An agent or pathogen can be indirectly transmitted from a reservoir to a susceptible host by inanimate objects or contaminated environment. Cleaning and disinfection are important practices to ensure that medical equipment surfaces do not serve as means of transmission for infectious pathogens. Hands of healthcare personnel may transmit pathogens after touching an infected or colonized body site on a patient or a contaminated inanimate object if hand hygiene is not performed before touching another patient (Siegel, 2007).
Patient-care devices, such as electronic thermometers, glucose monitoring devices, stethoscopes, blood-pressure cuffs, and other devices may transmit pathogens if they are contaminated with blood or bodily fluids or are shared between patients without cleaning and disinfecting. Shared toys may become a vehicle for transmitting respiratory viruses (eg, respiratory syncytial virus) or pathogenic bacteria (e.g., Pseudomonas aeruginosa) among pediatric patients (Siegel, 2007).
Toys used by young children should be washable. A system should ensure that they are washed and dried routinely. Older children should wash hands before and after using shared toys or equipment.
Instruments (e.g., endoscopes, surgical instruments) that are inadequately cleaned between patients before disinfection or sterilization or that have manufacturing defects that interfere with the effectiveness of reprocessing may transmit bacterial and viral pathogens. Clothing, uniforms, laboratory coats, or isolation gowns used as personal protective equipment (PPE) may become contaminated with potential pathogens after care of a patient colonized or infected with an infectious agent (Siegel, 2007).
Airborne Transmission
Transmission of germs can also occur through the airvia droplet or airborne routes. Droplet transmission is common, easily spreadinginfections such as colds, influenza, whooping cough (pertussis), and some forms of meningitis. Droplets are produced when the infected person coughs, sneezes, or speaks. Droplets can travel about 3 to 6 feet before drying out or falling to the ground. Droplet Precautions are designed to interrupt this means of transmission, and respiratory hygiene practices recommend that they be used when caring for any person with active respiratory symptoms.

This photograph captures a sneeze in progress, revealing the plume of salivary droplets as they are expelled in a large cone-shaped array from this man’s open mouth, thereby dramatically illustrating the reason for covering your mouth when coughing or sneezing in order to protect others from germ exposure. Source: CDC.
Airborne transmission occurs with only a few infections—those caused by organisms that can survive the drying of respiratory droplets. When the droplets evaporate, they leave behind droplet nuclei, which are so tiny they remain suspended in the air. Diseases transmitted by the airborne route include tuberculosis, chickenpox, measles, severe acute respiratory syndrome (SARS), and smallpox. Airborne Precautions are designed to interrupt this means of transmission.
Means of transmission that are not common in hospitals include:
- Common-source vehicles such as contaminated food, water, milk, or IV fluid. In hospitals, these products are obtained only from safe and approved sources to prevent contamination.
- Vector-borne transmission by an animal carrier such as a rat or mosquito that carries the pathogen from reservoir to host. Hospitals maintain their environment so that vector-borne transmission is not likely to occur.
Activities aimed at eliminating the means of transmission in healthcare facilities include:
- Hand hygiene
- Wearing gloves to minimize contamination of hands and discarding them after each patient
- Maintaining Standard, Contact, Droplet and Airborne Precautions as indicated
- Cleaning, disinfection, or sterilization of equipment used by more than one patient
- Cleaning of the environment, especially high-touch surfaces
- Maintaining directional air flow
Portals of Entry: How Pathogens Are Introduced
The portal of entry refers to the location through which a pathogen enters a susceptible host. The portal of entry must provide access to tissues in which the pathogen can multiply or a toxin can act. Often, the infectious agent uses the same portal to enter the new host that it used to exit the source host. For example, influenza virus exits the respiratory tract of the source host and enters the respiratory tract of the new host.
Other pathogens follow a so-called fecal-oral route because they exit the source host in feces, are carried on inadequately washed hands to a vehicle such as food, water, or utensils, and enter a new host through the mouth. Other portals of entry include skin, mucous membranes, and blood.
Pathogens cannot cause illness until they gain entry into the body, and, in general, they cannot enter through intact skin. They may gain entry through an anatomic opening, a skin break caused by illness or accident, or an opening created during a medical procedure, such as a surgical wound or an IV site. Preventing or eliminating portals of entry, where possible, and protecting portals that cannot be eliminated is a must for both patients and healthcare personnel.
Examples of portals of entry include:
- Mouth, nose, eyes, and other anatomic openings
- Rash or dermatitis
- Insect bites
- Injuries, from microscopic to major
- Surgical wounds
- Intravenous sites
- Any location, whether anatomic or created, with a tube in place
- Needle-puncture injuries
Activities aimed at protecting or eliminating portals of entry in healthcare facilities include:
- Use of aseptic surgical technique
- Application of dressings on surgical wounds
- Use of IV site dressings and proper care
- Elimination of tubes as soon as possible
- Use of masks, goggles, and face shields
- Protecting your skin to prevent holes (such as dermatitis)
- Keeping unwashed hands and objects away from the mouth
- Use of actions and devices to prevent needle sticks
Susceptible Host
The final link in the chain of infection is the susceptible host. Most of the factors that influence infection and the occurrence and severity of disease are related to the host, although agent and environmental factors also play a role (table below). However, characteristics of the host-agent interaction—such as pathogenicity, virulence, and antigenicity—are important. The infectious dose, mechanism of disease production, and route of exposure are also factors.
|
Factors That Influence the Outcome of an Exposure |
|
|---|---|
|
Host factors |
|
|
Agent factors |
|
|
Environmental factors |
|
Some people exposed to pathogenic microorganisms never develop symptomatic disease while others become severely ill and even die. Those who are extremely old or young, are already ill, have holes in their skin, have invasive devices in place, or are immunocompromised are more susceptible. Still others progress from colonization to symptomatic disease either immediately following exposure or after a period of asymptomatic colonization.
Susceptibility can be reduced in several ways. For some diseases there are effective vaccines and some diseases produce lasting immunity after illness. We have better resistance to disease when we are well rested, well fed, and relatively stress-free. People with healthy immune systems are often able to resist infection even when bacteria do invade.
The healthy body has numerous protective structures and systems that support resistance to infection. These include intact skin, blood circulation bringing white blood cells and nutrients to the tissues, antibodies to previously encountered infectious agents, the inflammatory response, stomach acid, and a robust community of normal flora, which provides competition to invading pathogens. A person with these defense mechanisms intact is said to be immunocompetent.
Immune compromise varies in severity and can be temporary or long term. A person who is sick in bed for a few days may be mildly compromised, while a person with a chronic illness such as diabetes is probably moderately and chronically compromised. Someone receiving chemotherapy or a transplant patient may be severely immunocompromised.
Extra care should be taken to protect a person who is immunocompromised. Nutritional status should be closely monitored to support immune competence. The care should be tailored to the specific needs and situation of the patient. Both the very young and very old need extra protection from infection. Any indwelling device (e.g., IV catheters, urinary catheters) increases susceptibility. To reduce the risk of infections associated with these devices, the device should be discontinued as soon as the patient no longer needs it.
Infections are sometimes more related to host factors than to the infectious agent. For example, a person who is well rested may resist the virus that makes the over-tired person sick. Some organisms are widely found but only cause disease in a susceptible host—such as the person recently treated with antibiotics who then develops a yeast infection. Examples of susceptible hosts include people who:
- Are already ill
- Have invasive devices or tubes in place
- Are malnourished
- Are very old or very young
- Are tired or under high stress
- Have skin breaks such as surgical wounds or IV sites
- Are undergoing steroid therapy or treatment for cancer
- Have HIV infection
- Are well and healthy! (No one is immune to all disease.)
Activities aimed at protecting or eliminating susceptible hosts in healthcare facilities include:
- Preventing exposure of both patients and staff to communicable disease
- Removal of invasive devices as soon as they are no longer needed
- Maintaining good nutrition
- Maintaining good skin condition
- Covering skin breaks
- Vaccinating people against illnesses to which they may be exposed
- Encouraging rest and balance in our lives
Prevention Strategies
Both science and regulation address prevention of HAIs. The CDC provides the chief authority for science. Regulations may be federal, state, or local.
Since 1991, when OSHA first issued its Bloodborne Pathogens Standard to protect healthcare personnel from blood exposure, the focus of regulatory and legislative activity has been on implementing a hierarchy of prevention and control measures. A central tenet is to consider all patients to be potentially infected with a bloodborne pathogen.
The federal OSHA Bloodborne Pathogens Standard requires that each employer having employees with occupational exposure to blood or other potentially infections material (OPIM) shall establish a written exposure control plan designed to eliminate or minimize employee exposure. Among other things, this plan must address:
- Standard/Universal Precautions, including hand hygiene (Element II)
- Engineering and work practice controls (See Element III.)
- Personal protective equipment (PPE) (See Element IV.)
- Housekeeping, laundry, regulated waste (See Element V.)
- Contaminated sharps and equipment (See Element III.)
- Hepatitis B vaccination and exposure followup (See element VI.)
- Employee communication and education (See Element VI.)
- Recordkeeping
The Exposure Control Plan must be available to employees. Many of the educational requirements are addressed in this course, but it does not cover employer-specific requirements such as how to report an exposure and it does not take the place of an employer-specific Exposure Control Plan.
The complete federal Bloodborne Pathogens Standard is available at the OSHA website.
Standard and Universal Precautions
Universal Precautions were originally developed by OSHA to protect healthcare workers from bloodborne pathogens, such as HIV, Hepatitis B (HBV), and hepatitis C (HCV). Universal Precautions were developed for use with all patients because those with bloodborne infections may be asymptomatic or unaware of their infectious status. Universal Precautions continue to be required by the OSHA Bloodborne Pathogens Standard.
Universal Precautions requires avoidance of contact with blood or OPIM. OSHA defines OPIM, or “other potentially infectious materials,” as (1) the following human body fluids: semen, vaginal secretions, cerebrospinal fluid, synovial fluid, pleural fluid, pericardial fluid, peritoneal fluid, amniotic fluid, saliva in dental procedures, any body fluid that is visibly contaminated with blood, and all body fluids in situations where it is difficult or impossible to differentiate between body fluids; (2) any unfixed tissue or organ (other than intact skin) from a human (living or dead); and (3) HIV-containing cell or tissue cultures, organ cultures, and HIV- or HBV-containing culture medium or other solutions; and blood, organs, or other tissues from experimental animals infected with HIV or HBV.
Because the focus of Universal Precautions was narrow (protect healthcare workers from bloodborne pathogens), the CDC was led to develop Standard Precautions, which include all of Universal Precautions and more.
Standard Precautions protect patients and healthcare workers from many bacterial and viral infections, including bloodborne pathogens. When we use Standard Precautions, we are in full compliance with Universal Precautions.
Standard Precautions tell us to avoid contact with:
- Blood and all body fluids from all patients. Any body fluid may carry microorganisms.
- Mucous membranes
- Non-intact skin (abrasions, dermatitis, rash)
Standard Precautions, as described by the CDC, requires all of the following:
- Assume that every person is potentially infected or colonized.
- Perform hand hygiene correctly and routinely.
- Use personal protective equipment (PPE) (see Element IV). Note that mask, eye protection, and gown may be required for care of a patient on Standard Precautions. Supplies must be readily available.
- Use safe injection practices to protect patients and workers—Elements II and III.
- Clean patient-care equipment and the environment as described in Element V.
- Implement respiratory hygiene in all areas where people with respiratory symptoms may be seen.
Respiratory hygiene was incorporated into Standard Precautions by the CDC in 2007, to:
- Educate healthcare personnel on the importance of source control measures to prevent droplet and fomite transmission of respiratory infection. Cover the portal of exit!
- Put these measures in place beginning at the point of initial encounter in the healthcare setting.
- Apply them to both patients and accompanying individuals.
- Post signs at entrances and other key locations asking people with respiratory symptoms to cover their mouths and noses when coughing or sneezing, to dispose of tissues and do hand hygiene. Signs in several languages are available from the CDC.
- Provide tissues, waste receptacles, and hand hygiene materials.
- Offer a simple mask to coughing patients.
- Encourage coughing patients to stay at least 3 feet away from others.
|
Correct Use of Standard Precautions |
|
|---|---|
|
Always |
|
|
Never |
|
Transmission-Based Precautions
The CDC also recommends, for patients with certain infections, use of transmission-based precautions, in addition to Standard Precautions. Standard Precautions are used with all patients and do not require a sign on the door. Patients being cared for using Contact, Droplet, or Airborne Precautions will have a sign on the door in most facilities. Note that the sign on the door may not specify the patient’s diagnosis for reasons of privacy.
Precautions may vary between facilities. Refer to your facility’s policies for details. Details for all types of precautions may be found in the CDC Guideline for Isolation Precautions, 2007. The list of diseases requiring transmission-based precautions and the duration of those precautions may be found in Appendix A of that guideline (CDC, 2010).
Facilities should have policies for transport of the patient outside the room, addressing each type of transmission-based precautions.
Contact Precautions
The following are CDC recommendations for acute care facilities. Other types of facilities should develop policies based on these guidelines:
- Hand hygiene is critical in the care of patients.
- Visitors must perform hand hygiene on leaving the room.
- Use a single-patient room if possible; consult with IC staff if not possible.
- Wear gloves to enter the room. Change gloves as specified by Standard Precautions.
- Wear a gown to enter the room.
- Use single-patient equipment, left in the room, as possible.
- Disinfect any equipment that must leave the room.
- Clean and disinfect these rooms at least daily.
Supplies needed include:
- Gloves
- Gowns
- Disinfectant for removed equipment
- Trash container for discarded PEP
- Single-patient use equipment
Contact Precautions are often used to care for patients with Methicillin-resistant Staphylococcus aureus, C. difficile, wounds with uncontained drainage, and a number of other infections.
Droplet Precautions
The following are CDC recommendations for acute care facilities. Other types of facilities should develop policies based on these guidelines:
- Use a single-patient room if possible; consult with IC staff if not possible.
- Maintain at least 3 feet separation between the patient and others.
- If two patients must share a room, draw the privacy curtain between them.
- Hand hygiene must be done as specified for Standard Precautions.
- Staff should wear a simple mask to enter the room.
- Supplies needed (beyond Standard Precautions) are limited to simple masks.
Droplet Precautions are used to provide care to patients with influenza, pertussis, some types of meningitis, undiagnosed respiratory infections, and several other diseases.
Airborne Precautions
Airborne Precautions are the only type that require:
- Airborne infection isolation room—AIIR—a negative pressure isolation room
- N-95 respirator or PAPR.
- Supplies needed (beyond Standard Precautions) are limited to the appropriate respiratory protection.
AIIRs have very specific requirements and are often available only in acute care facilities. If a disease requiring Airborne Precautions is suspected and an AIIR is not available, place a simple mask on the patient and place him/her in a separate room with its door closed while transfer to a facility with an available AIIR is arranged. Non-acute care settings should have well known policies for identifying and managing such patients.
If patients must come out of the AIIR, put a simple mask on them; a tight-fitting respirator may not be tolerated and is not indicated.
Airborne Precautions are used for patients known or suspected of having:
- Tuberculosis, active pulmonary or rule-out
- Chickenpox
- Measles
- Disseminated herpes zoster (shingles of more than one dermatome)
- SARS
- Smallpox
Tuberculosis
Every year, more than 9 million people worldwide develop tuberculosis (TB) and nearly 2 million people die from the disease. Tuberculosis is a bacterial infection caused by Mycobacterium tuberculosis and is spread in airborne droplets when people with the disease cough or sneeze. Most people with healthy immune systems infected with M. tuberculosis never become ill. However, the bacteria remain dormant within the body and can cause tuberculosis years later if host immunity declines.
The person who is most likely to transmit tuberculosis is the person who has not been diagnosed—the unknown carrier. Identification without delay of the person with active tuberculosis is critical so that isolation and treatment can prevent transmission to others.
Active TB does have symptoms, which depend on where in the body the TB bacteria are growing. Tubercular disease in the lungs may cause symptoms such as a bad cough that lasts 3 weeks or longer, pain in the chest, or coughing up blood or sputum (phlegm from deep inside the lungs). Other symptoms of active TB disease are weakness or fatigue, weight loss, no appetite, chills, fever, or sweating at night (CDC, 2005).
Diagnostic tests for the disease include chest x-rays, the tuberculin skin test, and sputum cultures. Tuberculosis can usually be cured by taking several powerful antibiotics daily for several months.
Because tuberculosis is the primary disease transmitted by a true airborne route, and because it is the undiagnosed person who is most likely to transmit disease, the CDC recommends a three-level hierarchy of controls: administrative, environmental, and respiratory.
Administrative controls specify who is in charge of the facility’s TB control program, including critical infrastructure such as laboratories as well as other services needed to maintain an effective program. A key component is having a plan to ensure prompt detection, airborne precautions, and treatment of persons who have suspected or confirmed TB disease. Diagnose, isolate, and treat to prevent exposing others. Environmental controls are provided to contain the source of exposure, primarily by the use of Airborne Infection Isolation (AII) rooms that provide negative-pressure ventilation.
Powered Air-Purifying Respirator Hood (PAPR)
Respiratory controls address the protection of people who must be protected from contaminated air when they enter the AII room. Most facilities provide N-95 respirators (see Module 6), which must be fit-tested. Some facilities exclusively use powered air-purifying respirators (PAPRs, see image at right) for all staff; they do not require fit testing. Check your facility’s policies for what respiratory protection is made available for visitors.
Tuberculosis infectiousness usually declines within weeks of beginning treatment. The patient must show clear clinical improvement before isolation is discontinued because the patient with resistant organisms remains infectious if initial treatment is not effective. Airborne Precautions for tuberculosis may be discontinued when both of the following criteria have been met: (1) clinical improvement, and (2) three consecutive sputum smears negative for acid-fast bacilli (TB germs).
Each of the three sputum specimens should be collected in eight 24-hour intervals and at least one specimen should be an early morning specimen.
For current guidelines, consult CDC Guidelines for Preventing the Transmission of Mycobacterium tuberculosis in Health-Care Settings.
Multidrug-resistant TB (MDR-TB) and extensively drug-resistant tuberculosis (XDR-TB) have become more common and are highly infectious. Treatment of drug-resistant TB is much more difficult than normal tuberculosis, requiring even more antibiotics, and for long periods, up to 2 years and beyond. In addition, because HIV weakens the immune system, HIV-positive people are much more likely to develop active tuberculosis (and to die from the disease, which speeds the development of AIDS) than people with a healthy immune system (Escombe et al., 2008).
Hand Hygiene
The term hand hygiene includes both the use of an alcohol-based hand rub and washing with soap and water.
The chain of infection makes it clear why hand hygiene is critical. For generations, handwashing with soap and water has been the standard measure of personal hygiene. The concept of cleansing hands with an antiseptic agent probably emerged in the early nineteenth century. As early as 1822, a French pharmacist demonstrated that solutions containing chlorides of lime or soda could eradicate the foul odors associated with human corpses and that such solutions could be used as disinfectants and antiseptics. In a paper published in 1825 this pharmacist stated that physicians and other persons attending patients with contagious diseases would benefit from moistening their hands with a liquid chloride solution (CDC, 2002).
CDC’s 2002 Guidelines for Hand Hygiene brought a major change in hand hygiene practices. While washing with soap and water is still required in some situations, now the use of an alcohol-based hand rub is preferred for routine use.
Despite the simplicity and effectiveness of hand hygiene in preventing the spread of infectious disease, adherence to hand hygiene practice remains unacceptably low throughout the world. Although measuring hand hygiene adherence is not a simple task, an oft-cited study by Pittet (2001) noted that adherence varies among professional categories of healthcare workers and between hospital departments but is usually estimated as less than 50%.
CDC (2002) has described observed and self-reported factors that influence adherence to hand hygiene practices.
Observed risk factors for poor adherence
- Physician (rather than nurse)
- Nursing assistant (rather than nurse)
- Male gender
- Working in intensive care unit
- Working during the week (rather than weekend)
- Wearing gowns/glove
- Automated sink
- Activities with high risk of cross contamination
- High number of hand hygiene opportunities per hour of patient care
Self-reported factors for poor adherence
- Handwashing agents cause irritation and dryness
- Sinks inconveniently located
- Lack of soap and paper towels
- Too busy
- Understaffing or overcrowding
- Patient needs take priority
- Hand hygiene interferes with patient/healthcare worker relationship
- Low risk of acquiring infection from patient
- Believing that wearing gloves means hand hygiene is unnecessary
- Lack of knowledge
- Forgetfulness
- Lack of role model
- Skepticism about need for hand hygiene
- Disagreement with recommendations
- Lack of scientific data to back up need for hand hygiene
For healthcare workers, adherence to hand hygiene guidelines protects both the patient and the worker. Hand hygiene should be done when:
- You first come on duty
- Before you touch your first patient or clean equipment
- Before and after every patient contact, including after touching intact skin
- Before any clean or invasive procedure
- Before putting on sterile gloves
- Before contact with any portal of entry, your patient’s or your own
- After contact with any body fluids, including your own
- After unprotected contact with mucous membranes or non-intact skin
- Each time you remove your gloves
- When leaving an isolation room
- When going from a dirtier to a cleaner part of the patient
- When your hands feel or look dirty
- After contact with contaminated things or environments, such as charts
- After handling used equipment or linen
- After using the bathroom
- Before and after eating
- Before going off duty
My 5 Moments for Hand Hygiene
The My 5 Moments for Hand Hygiene approach defines the key moments when healthcare workers should perform hand hygiene. This evidence-based, field-tested, user-centered approach is designed to be easy to learn, logical, and applicable in a wide range of settings (Sax, 2007).
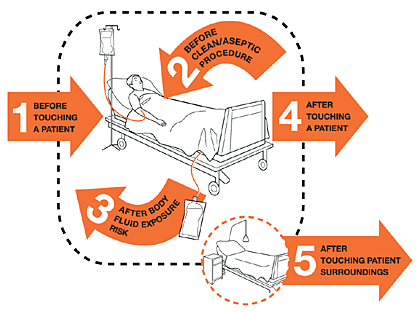
Source: Copyright © World Health Organization, 2009. All rights reserved.
Choosing Alcohol Sanitizer or Soap and Water
If you can see dirt on your hands—whether from blood, body fluid, or other visible soiling—wash your hands with soap and water, which physically removes the dirt from your hands. Washing with soap and water does not kill germs.
Alcohol hand rubs do kill most germs including viruses, but they do not remove dirt and debris from your hands. If you use alcohol, choose a hand hygiene product that contains alcohol; plain alcohol should not be used because it evaporates too quickly to provide enough contact time to kill germs.
How to do hand hygiene right:
- If using soap, wet your hands first to minimize skin irritation.
- Use friction on all surfaces to loosen dirt and germs.
- Scrub for at least 15 seconds (Row, Row, Row Your Boat, twice).
- Use a comfortable water temperature; water hot enough to kill germs would injure your skin.
- Use alcohol hand rubs on dry skin only.
- Use one measured amount of alcohol sanitizer, and rub until hands are completely dry; do not wipe off with a paper towel.
For routine hand hygiene, alcohol products are preferred. They are better than soap and water because:
- They do kill germs
- They leave skin in better condition
- They are quicker and easier, so are used more frequently
When dealing with diarrhea that may be infectious, use soap and water. Both Clostridium difficile and norovirus cause diarrhea and neither is effectively killed by alcohol-based hand rubs.
Because alcohol products are effective antimicrobial agents, the CDC does not specify an antimicrobial soap for routine hand hygiene. Antimicrobial soaps are often more irritating to the skin, are more expensive, and tend to build up in the environment. “Plain” soap removes germs from the hands as well as an antimicrobial product.
Safe Injection Practices: Protecting Patients
[This section derived largely from NYSDOH, 2011.]
Needles, cannulae, and syringes are sterile, single-use items—any use will result in these items being contaminated. They are contaminated once they are used to enter or connect to any component of a patient’s intravenous infusion set. After use, immediately dispose of all needles and syringes into a leak-proof, puncture-resistant, closable container. Develop policies and procedures to prevent sharps injuries among staff and review them regularly.
Medications and Solutions
A pathogen can be indirectly transmitted through contaminated medications and injection equipment. For this reason, medications and solutions must be properly handled whether they are single or multidose. To prevent cross contamination, preparation and disposing of medications should be handled in areas designated for that purpose.
The reuse of needles or syringes and the misuse of medication vials are serious threats to public health. Healthcare providers should never reuse a needle or syringe, either from one patient to another or to withdraw medicine from a vial. Both needle and syringe must be discarded once they have been used. It is not safe to change the needle and reuse the syringe—reuse of needles or syringes to access medication can result in contamination of the medicine with infectious material that can be spread to others when the medicine is used again (CDC, 2011).
Injectable Device
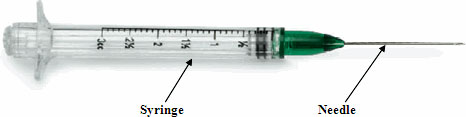
Neither portion of the injectable device may be reused under any circumstances. Source: CDC.
Single-Use Vials
A single-use vial is a bottle of liquid medication that is given to a patient through a needle and syringe. Single-use vials contain only one dose of medication and should only be used once for one patient, using a clean needle and clean syringe. Use single-dose vials for parenteral medications whenever possible. Do not administer medications from single-dose vials or ampoules to multiple patients or combine leftover contents for later (CDC, 2011).
Multidose Vials
A multidose vial is a small sealed container holding more than one dose of medication, vaccine, or fluid. The advantages of multidose vials include being able to adjust dosage of medication easily, less waste of left-over medication, cost savings in packaging, and ease of use. For the medication to remain sterile and safe for use between patients, a new sterile needle and syringe must be used every time the vial is entered.
A review of HBV and HCV outbreak information in 12 outpatient clinics, 16 hemodialysis centers, and 15 long-term care facilities revealed that 448 people became infected with HBV or HCV between 1998 and 2008 as a result of poor infection control practices or failure to use aseptic technique (Thompson et al., 2009).
To prevent these sorts of breaches, minimize the use of multidose vials whenever possible. If multidose vials must be used, always use aseptic technique. Use a new needle or cannula and a new syringe to access the multidose vial. Do not keep the vials in the immediate patient treatment area. Do not use bags or bottles of IV solution as a common source of medication or fluid for multiple patients. Use infusion sets (i.e., intravenous bags, tubing, and connectors) for one patient only and dispose appropriately after use.
Aseptic Technique
Aseptic technique involves the handling, preparation, and storage of medications in a manner that prevents microbial contamination. It also applies to the handling of all supplies used for injections and infusions, including syringes, needles, and IV tubing. To avoid contamination, medications should be drawn up in a clean medication preparation area. Any item that may have come in contact with blood or bodily fluids should be kept separate from medications. Incorrect practices that have resulted in transmission of hepatitis C or hepatitis B virus include using:
- The same syringe to administer medication to more than one patient, even if the needle was changed
- The same medication vial for more than one patient, and accessing the vial with a syringe that has already been used to administer medication to a patient
- A common bag of saline or other IV fluid for more than one patient, and accessing the bag with a syringe that has already been used to flush a patient’s catheter
In addition to strictly adhering to aseptic technique, ensure that all staff perform proper hand hygiene before and after gloving, between patients, and whenever hands are soiled. Avoid cross contamination with soiled gloves. Provide adequate soap and water, disposable paper towels, and waterless alcohol-based hand rubs throughout all medical facilities.
Preventing Disease Transmission
Safe injection practices are designed to prevent disease transmission from patient to patient and healthcare worker to patient. The absence of visible blood or signs of contamination in a used syringe, IV tubing, multidose medication vial, or blood glucose monitoring device does not mean the item is free from potentially infectious agents. Bacteria and other microbes can be present without clouding or other visible evidence of contamination. All used injection supplies and materials are potentially contaminated and should be discarded.
Many cases reported to the CDC in which a bloodborne pathogen was transmitted as a result of improper injection practices have common themes and findings. Often aseptic technique and Standard Precautions were not carefully followed. In many cases infection control programs were lacking or responsibilities were unclear. Lack of recognition of an IC breach led to prolonged transmission and a growing number of infected patients. In all cases, investigations were time-consuming and costly and required the notification, testing, and counseling or hundreds and sometimes thousands of patients.
To ensure safe injection practices, providers should use aseptic technique throughout all aspects of injection preparation and administration. Medications should be drawn up in a designated “clean” medication area that is not adjacent to areas where potentially contaminated items are placed. In addition:
- Use a new sterile syringe and needle to draw up medications for each patient. Prevent contact between the injection materials and the non-sterile environment.
- Practice proper hand hygiene before handling medications.
- Disinfect the rubber septum of a medication vial with alcohol prior to piercing.
- Discard medication vials upon expiration or any time there are concerns regarding the sterility of the medication.
Never leave a needle or other device inserted into a medication vial septum, IV bag, or bottle for multiple uses. This provides a direct route for microorganisms to enter the vial and contaminate the fluid. Medications should never be administered from the same syringe to more than one patient, even if the needle is changed. Never use the same syringe or needle to administer IV medications to more than one patient, even if the medication is administered into the IV tubing, regardless of the distance from the IV insertion site.
Keep in mind that all of the infusion components from the infusate to the patient’s catheter are a single interconnected unit. All of the components are directly or indirectly exposed to the patient’s blood and cannot be used for another patient. Syringes and needles that intersect through any port in the IV system also become contaminated and cannot be used for another patient or used to re-enter a nonpatient-specific multidose vial. Separation from the patient’s IV by distance, gravity, or positive infusion pressure does not ensure that small amounts of blood are not present in these items.
Dedicate vials of medication to a single patient. Never enter a vial with a syringe or needle that has been used for a patient if the same medication vial might be used for another patient. Medications packaged as single-use must never be used for more than one patient. Never combine leftover contents for later use. Medications packaged as multi-use should be assigned to a single patient whenever possible. Never use bags or bottles of IV solution as a common source of supply for more than one patient.
Peripheral capillary blood monitoring devices packaged for single-patient use should never be used on more than one patient. Restrict use of peripheral capillary blood sampling devices to individual patients. Never reuse lancets. Consider selecting single-use lancets that permanently retract upon puncture. Whenever possible evaluate and select safer devices to prevent sharps injuries. In 2012 FDA, NIOSH, and OSHA issued a joint safety communication. This document strongly encourages healthcare professionals to use blunt-tip suture needles as an alternative to standard suture needles when suturing fascia and muscle to decrease the risk of needlestick injury (CDC, 2014b).
Back Next
