The use of engineering and work practice controls to reduce the opportunity for patient and healthcare worker exposure to potentially infectious material should be standard practice in all healthcare settings, not only in hospitals. Facilities are required to address and manage high-risk practices and procedures capable of causing healthcare-acquired infections (HAIs) from bloodborne pathogens. (updated guideline)
[The following information is taken from the OSHA Bloodborne Pathogens Standard, 1910.1030.]
Engineering controls such as sharps disposal containers, self-sheathing needles, and safer medical devices (sharps with engineered sharps injury protections and needleless systems) isolate or remove the hazard from the workplace.
Work practice controls reduce the likelihood of exposure by altering the manner in which a task is performed (e.g., prohibiting recapping of needles by a two-handed technique).
Engineering and work practice controls are intended to eliminate or minimize employee exposure. They must be examined and maintained or replaced on a regular schedule to ensure their effectiveness. Engineering controls usually involve an object, such as a safer chemical, syringe with engineered safety protection, sharps container, or splash guard. Work practice controls reduce risk by altering the way a task is performed. Work practice controls tell how to do the job safely, and should be described in written procedures. Engineering and work practice controls are designed to reduce risk of percutaneous, mucous membrane/non-intact skin or parenteral exposures of workers.
Percutaneous (through the skin) exposures can occur during handling, disassembly, disposal, and reprocessing of contaminated needles and other sharp objects, or via human bites, cuts, and abrasions.
Activities that risk percutaneous exposures include manipulating contaminated needles and other sharp objects by hand, removing scalpel blades from holders, and removing needles from syringes.
Delaying or improperly disposing of sharps, leaving contaminated needles or sharp objects on counters or workspaces, or disposing of sharps in nonpuncture-resistant receptacles can lead to injury. Recapping contaminated needles and other sharp objects using a two-handed technique is a common cause of injury. Percutaneous exposures can also occur when performing procedures where there is poor visualization—such as blind suturing, non-dominant hand positioned opposed or next to a sharp, and performing procedures where bone spicules or metal fragments are produced.
Mucous membrane/non-intact skin exposures occur when there is direct blood or body fluids contact with the eyes, nose, mouth, or other mucous membranes. This can occur via contact with contaminated hands, contact with open skin lesions/dermatitis, and from splashes or sprays of blood or body fluids (e.g., during irrigation or suctioning).
Parenteral refers to a route of transmission or administration that involves piercing mucous membranes or the skin barrier through such events as needlesticks, human bites, cuts, and abrasions. A parenteral exposure occurs as a result of injection with infectious material, which can occur during administration of parenteral medication, sharing of blood monitoring devices such as glucometers, hemoglobinometers, lancets, and lancet platforms/pens, and infusion of contaminated blood products or fluids.
According to OSHA, nurses sustain the most needlestick injuries, and as many as one-third of all sharps injuries occur during disposal. The CDC estimates that 62% to 88% of sharps injuries can be prevented simply by using safer medical devices (OSHA, 2012).
Sharps Safety: Protecting Healthcare Workers
[This section is derived from CDC, 2008.]
There has been increased focus on removing sharps hazards through the development and use of engineering controls. In November 2000 the Federal Needlestick Safety and Prevention Act authorized OSHA’s revision of its Bloodborne Pathogens Standard to require the use of safety-engineered sharp devices (see below). The CDC has provided guidance on the design, implementation, and evaluation of a comprehensive sharps injury prevention program. This includes measures to handle needles and other sharp devices in a manner that will prevent injury to the user and to others who may encounter the device during or after a procedure.
Healthcare workers must follow proper technique when using and handling needles, cannulae, and syringes. Whenever possible, use sharps with engineered sharps injury protections—for example, non-needle sharp or needle devices with built-in safety features or mechanisms that effectively reduce the risk of an exposure incident.
Always activate safety features—do not circumvent them. Modify procedures if necessary to avoid injury. For example:
- Use forceps, suture holders, or other instruments for suturing.
- Avoid holding tissue with fingers when suturing or cutting.
- Avoid leaving exposed sharps of any kind on patient procedure or treatment work surfaces.
- Use appropriate safety devices whenever available.
Sharps with Safety Features Exposed (left) and Covered (right)
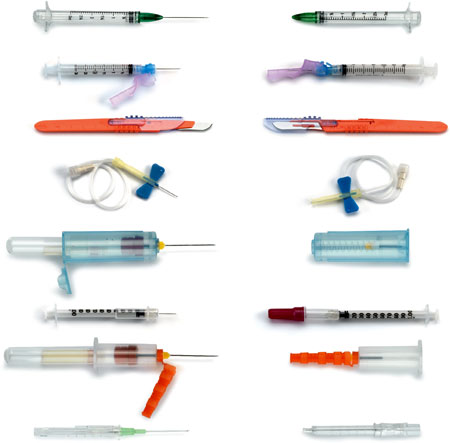
Source: CDC.
In surgical and obstetrical settings where the use of exposed sharps cannot be avoided, work-practice controls are an important adjunct for preventing blood exposures, including percutaneous injuries. Operating room controls include:
- Using instruments, rather than fingers, to grasp needles, retract tissue, and load/unload needles and scalpels
- Giving verbal announcements when passing sharps
- Avoiding hand-to-hand passage of sharp instruments by using a basin or neutral zone
- Using alternative cutting methods such as blunt electrocautery and laser devices when appropriate
- Substituting endoscopic surgery for open surgery when possible
- Using round-tipped scalpel blades instead of sharp-tipped blades (CDC, 2004)
The use of blunt suture needles, an engineering control, is also shown to reduce injuries. These measures help protect both the healthcare provider and the patient from exposure to the other’s blood.
Puncture-resistant containers located at the point of use are used to discard sharps, including needles and syringes, scalpel blades, unused sterile sharps, and discarded slides or tubes with small amounts of blood. To prevent needlestick injuries, needles and other contaminated sharps should not be recapped, purposefully bent, or broken by hand.
As part of their responsibility for providing a safe workplace, employers must provide handwashing facilities that are readily accessible to employees. If it is not feasible to provide handwashing facilities, the employer must provide antiseptic hand cleanser and clean cloth or paper towels, or antiseptic towelettes. When antiseptic hand cleansers or towelettes are used, hands should be washed with soap and running water as soon as possible.
Work Practice Controls: How to Do the Job Safely
[This section is taken largely from OSHA, 2012.]
Contaminated needles and other contaminated sharps should not be bent, recapped, or removed unless the employer can demonstrate that there is no alternative or that such action is required by a specific procedure. Any required manipulation must be accomplished through the use of a mechanical device or a one-handed technique. Shearing or breaking of contaminated needles is prohibited.
Immediately, or as soon as possible after use, contaminated reusable sharps must be placed in appropriate containers until properly reprocessed. These containers must be:
- Puncture resistant
- Labeled or color-coded in accordance with this standard
- Leakproof on the sides and bottom
- Maintained in accordance with OSHA requirements for reusable sharps
Eating, drinking, smoking, applying cosmetics or lip balm, and handling contact lenses are prohibited in work areas where there is a reasonable likelihood of occupational exposure. Food and drink should not be kept in refrigerators, freezers, shelves, cabinets, or on countertops or bench tops where blood or OPIM are present. It may be useful to designate areas to be kept free of body fluids (no specimens or gloves) where drinks may be permitted.
All procedures involving blood or OPIM must be performed in such a manner as to minimize splashing, spraying, spattering, and generation of droplets of these substances. Mouth pipetting or suctioning of blood or OPIM is prohibited.
Specimens of blood or OPIM must be placed in a container that prevents leakage during collection, handling, processing, storage, transport, or shipping. The container must be labeled or color-coded according to OSHA guidelines. When a facility utilizes Standard Precautions in the handling of all specimens, the labeling or color-coding of specimens is not necessary provided containers are recognizable as containing specimens, although this exemption only applies while such specimens or containers remain within the facility. Labeling or color-coding is required when such specimens or containers leave the facility.
If outside contamination of the primary container occurs, the primary container must be placed within a second container that prevents leakage during handling, processing, storage, transport, or shipping, and is labeled or color-coded according to the requirements of this standard. If the specimen could puncture the primary container, the primary container shall be placed within a secondary container that is puncture-resistant in addition to the above characteristics.
Equipment that may become contaminated with blood or other potentially infectious materials must be examined before servicing or shipping and be decontaminated as necessary, unless the employer can demonstrate that decontamination of such equipment or portions of such equipment is not feasible. A readily observable label must be attached to the equipment stating which portions remain contaminated.
The employer must ensure that this information is conveyed to all affected employees, the servicing representative, and the manufacturer before handling, servicing, or shipping, so that appropriate precautions will be taken.
Use splatter shields on medical equipment associated with risk-prone procedures (e.g., locking centrifuge lids). Gloves used for the task of sorting laundry should be of sufficient thickness to minimize sharps injuries.
General Practices for the Workplace
- Use proper hand hygiene, including the appropriate circumstances in which alcohol-based hand sanitizers and soap-and-water handwashing should be used.
- Use proper procedures for cleaning of blood and bodily fluid spills, including initial removal of bulk material followed by disinfection with an appropriate disinfectant.
- Practice proper handling and disposal of blood and bodily fluids, including contaminated patient care items.
- Select, put on, take off, and dispose of PPE as trained.
- Protect work surfaces in direct proximity to patient procedure treatment areas with appropriate barriers to prevent instruments from becoming contaminated with bloodborne pathogens.
- Prevent percutaneous exposures by avoiding unnecessary use of needles and other sharp objects.
- Use care in the handling and disposing of needles and other sharp objects.
Evaluation/Surveillance of Exposure Incidents
Employers must identify those at risk for exposure and what devices cause exposure. All sharp devices can cause injury and disease transmission if not used and disposed of properly. For example, hollow-bore needles have a higher disease transmission risk, while butterfly-type IV catheters, devices with recoil action, and blood glucose monitoring devices (lancet platforms/pens) have a higher injury rate.
Areas or Settings Where Exposures Occur
Sharps injuries don’t just occur in hospitals and labs—they can occur in other healthcare settings, such as nursing homes, clinics, emergency care services, and private homes. Although it is estimated that more than 350,000 sharps injuries occur each year in the United States, the CDC estimates 50% or more of healthcare personnel do not report occupational percutaneous injuries (CDC, 2008). Six sharps devices are responsible for nearly 80% of all injuries. These are:
- Disposable syringes (30%)
- Suture needles (20%)
- Winged steel needles (12%)
- Scalpel blades (8%)
- Intravenous (IV) catheter stylets (5%)
- Phlebotomy needles (3%)
Devices requiring manipulation or disassembly after use (such as needles attached to IV tubing, winged steel needles, and IV catheter stylets) are associated with a higher rate of injury than the hypodermic needle or syringe. Injuries from hollow-bore needles, especially those used for blood collection or IV catheter insertion, are of particular concern. These devices are likely to contain residual blood and are associated with an increased risk for HIV transmission. Overall, hollow-bore needles are responsible for 56% of all sharps injuries (CDC, 2008).
The largest percentage (39%) of sharps injuries occur on inpatient units, particularly medical floors and intensive care units (ICUs). The operating room is the second most common environment in which sharps injuries occur, accounting for 27% of injuries overall. Injuries most often occur:
- After use and before disposal of a sharp device (40%)
- During use of a sharp device on a patient (41%)
- During or after disposal (15%) (CDC, 2008)
Although nurses sustain the highest number of percutaneous injuries, other patient-care providers, laboratory staff, and support personnel are also at risk. Nurses are the predominant occupational group injured by needles and other sharps, in part because they are the largest segment of the workforce at most hospitals (CDC, 2008).
Back Next