*This course has been retired. There is no replacement course at this time. Please click here to view the current ATrain course listings.
A vaccine is a substance (an antigen) made from a virus or bacterium that triggers the body’s immune system to develop antibodies. Substances are sometimes added to a vaccine to generate a stronger immune response so that less vaccine is needed for the body to recognize and fight the antigen. Influenza vaccines cause antibodies to develop about 2 weeks after vaccination.
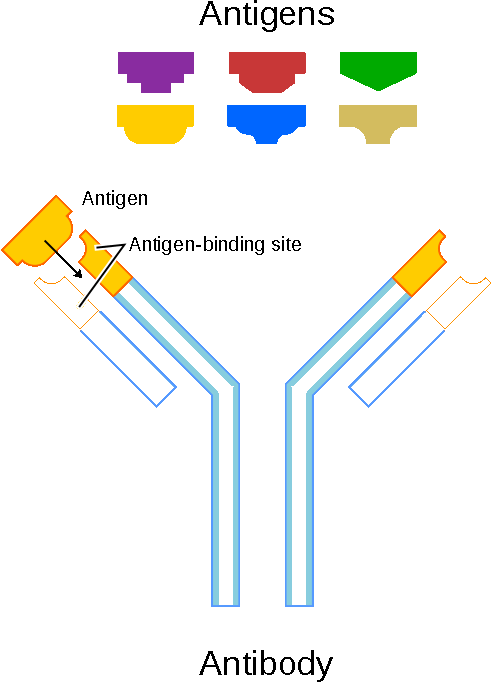
Schematic diagram of an antibody and antigens. Source: National Human Genome Research Institute.
Influenza vaccines are available in an inactivated form (IIV) and a live attenuated form (LAIV). Inactivated influenza vaccines have been available since the 1940s and have traditionally been administered intramuscularly or intradermally. Live attenuated vaccine was approved for use in the United States in 2003. Live attenuated influenza vaccines contain a version of the living microbe that has been weakened in the lab so it cannot cause disease.
Inactivated vaccines are produced by killing the disease-causing microbe in a virus or bacteria with chemicals, heat, or radiation. Inactivated vaccines are more stable and safer than live vaccines because the dead microbes cannot mutate back to their disease-causing state. However, most inactivated vaccines stimulate a weaker immune system response than do live vaccines (NAIAD, 2019b).
Influenza Vaccine Key Points | |
|---|---|
Inactivated influenza vaccine (IIV) |
|
Live attenuated vaccine (LAIV) |
|
Influenza Vaccine Production Technologies
In late February to early March—well before the new flu season begins—an FDA advisory committee reviews data about which flu viruses have caused disease in the past year, how the viruses are changing, and disease trends, so they can recommend the three or four flu strains to include in the trivalent and quadrivalent influenza vaccines for the United States in the upcoming flu season (FDA, 2019a). There are three different influenza vaccine production technologies approved by the FDA:
- Egg-based flu vaccine
- Cell-based flu vaccine
- Recombinant flu vaccine (2018c)
The most common way that flu vaccines are made is using an egg-based manufacturing process that has been used for more than 70 years. Egg-based vaccine manufacturing is used to make both inactivated (killed) vaccine used in the flu shot and live attenuated (weakened) vaccine used in the nasal spray flu vaccine (NIAID, 2019a).
Cell-based production of flu vaccines was approved by the FDA in 2012. Until recently, this production process began with egg-grown CVVs (candidate vaccine viruses). However, on August 31, 2016, the FDA issued an approval for Seqirus, the sole FDA-approved cell-based flu vaccine manufacturer in the United States, to use CVVs that are grown in animal cells. Cell culture technology has the potential for a faster start-up of the flu vaccine manufacturing process (NIAID, 2019b).
Recombinant technology for flu vaccines was approved for the U.S. market in 2013. It does not require an egg-grown vaccine virus and can be produced in a shorter amount of time than either egg-grown or cell-grown vaccine viruses.
Manufacturers using recombinant technology isolate a certain gene (the hemagglutinin or HA gene) from a naturally occurring (wild type) recommended vaccine virus. This HA gene is combined with portions of another virus that grows well in insect cells. It is then mixed with insect cells and allowed to replicate. The flu HA protein is then harvested from these cells and purified. Currently, recombinant flu vaccine is the only 100% egg-free vaccine on the U.S. market (CDC, 2018c).
The only influenza vaccine produced using recombinant technology is Flublok Quadrivalent. It has been licensed by the FDA for use in adults 18 years and older (CDC, 2018).
Trivalent Inactivated Vaccines (IIV3)
For years, flu vaccines were designed to protect against three different flu viruses (trivalent vaccines). Standard dose trivalent vaccines include an influenza A (H1N1) virus, an influenza A (H3N2) virus and one influenza B virus. Because there are two distinct lineages of influenza B viruses—Victoria and Yamagata—immunization against a single influenza B virus provides only limited cross protection against strains in the other lineage.
The standard dose trivalent shot (IIV3), is manufactured using virus grown in eggs. This shot (Afluria) can be given either with a needle (for people aged 5 years and older) or with a jet injector (or people aged 18 through 64 years only) (CDC, 2019p).
A three-component (trivalent) inactivated flu vaccine called Fluzone High-Dose is licensed specifically for people 65 years and older. Fluzone High-Dose contains four times the antigen of standard-dose inactivated influenza vaccines. The higher dose of antigen in the vaccine is intended to give older people a better immune response, and therefore, better protection against flu (CDC, 2019e).
The high-dose vaccine has been approved for use in the United States since 2009. Results of a clinical trial of more than 30,000 participants showed that adults 65 years and older who received the high-dose vaccine had 24% fewer influenza infections compared to those who received the standard dose flu vaccine (CDC, 2019m).
Fluzone
Fluzone, Fluzone High-Dose, Fluzone Intradermal Quadrivalent, and Fluzone Quadrivalent are all injectable vaccines. The intradermal flu vaccine is a shot that is injected into the skin instead of the muscle. The intradermal shot uses a much smaller needle than the regular flu shot, and it requires less antigen to be as effective as the regular flu shot. It may be used in adults 18 to 64 years of age.
Source: FDA, 2019b.
A trivalent flu shot made with adjuvant (Fluad) is approved for people 65 years and older. An adjuvant is an ingredient added to a vaccine to create a stronger immune response. The vaccine, FLUAD (allV3), was licensed in November 2015 and became available during the 2016–2017 flu season. It contains MF59 adjuvant, an oil-in-water emulsion of squalene oil. FLUAD is the first seasonal flu vaccine with adjuvant marketed in the United States. Squalene, a naturally occurring substance found in humans, animals, and plants, is highly purified for the vaccine manufacturing process (CDC, 2019b).
Quadrivalent Vaccines
Most influenza vaccines available in the 2020-2021 season will be quadrivalent vaccines. The quadrivalent flu vaccine is designed to protect against four different flu viruses: two influenza A viruses and two influenza B viruses. Adding another B virus to the vaccine aims to give broader protection against circulating flu viruses (CDC, 2019n).
Standard-dose quadrivalent flu shots are manufactured using virus grown in eggs. These include Afluria Quadrivalent, Fluarix Quadrivalent, FluLaval Quadrivalent, and Fluzone Quadrivalent. Different flu shots are approved for different age groups. There is a quadrivalent flu shot that can be given to children as young as 6 months of age. Other quadrivalent flu shots are approved for people 3 years and older (CDC, 2019n).
Most flu shots are given in the arm muscle with a needle. One quadrivalent flu shot (Afluria Quadrivalent) can be given either with a needle (for people aged 5 years and older) or with a jet injector (for people aged 18 through 64 years only) (CDC, 2019b).
A quadrivalent cell-based flu shot (Flucelvax Quadrivalent) containing virus grown in cell culture, is approved for people 4 years and older. A recombinant quadrivalent flu shot (Flublok Quadrivalent) is approved for people 18 years and older (CDC, 2019).
2018 Flublok Recombinant Influenza Vaccine (RIV4)
In 2013 the U.S. Food and Drug Administration (FDA) announced its approval of Flublok, a trivalent inactivated influenza vaccine for the prevention of seasonal influenza in people 18 years and older. Flublok’s manufacturing process has the potential for faster startup of vaccine manufacturing, which can be useful in the event of a pandemic or vaccine supply shortage, mainly because it is not dependent on an egg supply or limited by the selection of vaccine viruses that are adapted for growth in eggs. Also, this vaccine is suitable for vaccinating people with egg allergies because it is not made using eggs.
Flublok Quadrivalent (RIV4) is available for the 2019–2020 influenza season. RIV4 is indicated for persons aged ≥18 years. RIV4 is manufactured without the use of influenza viruses; therefore, similarly to IIVs, no shedding of vaccine virus will occur. RIV4 is produced without the use of eggs, and thus is egg-free. No preference is expressed for RIV4 versus IIVs within specified indications. RIV4 is administered by intramuscular injection (Grohskopf et al., 2018), CDC, 2019o).
Live Attenuated Influenza Vaccine (LAIV)
Live attenuated influenza vaccine (LAIV) was approved for use in the United States in 2003. LAIV contains the same influenza viruses as inactivated influenza vaccines. It does not contain thimerosal or any other preservative.
LAIV is provided in a single-dose sprayer unit; half of the dose is sprayed into each nostril. The nasal spray flu vaccine contains attenuated (weakened) live viruses that will not cause influenza illness. The weakened viruses are cold-adapted, which means they are designed to only multiply at the cooler temperatures found within the nose. The viruses cannot infect the lungs or other areas where warmer temperatures exist.
During previous flu seasons (2016–2017 and 2017–2018), the Advisory Committee on Immunization Practices (ACIP) recommended that LAIV4 not be used because of concerns about low effectiveness against influenza A(H1N1)pdm09-like viruses circulating in the United States during the 2013–2014 and 2015–2016 seasons (Grohskopf et al., 2018).
For the 2019–2020 flu season, ACIP recommends any influenza vaccine that is appropriate for the recipient’s age and health status, including inactivated influenza vaccine (IIV), recombinant influenza vaccine (RIV), or live attenuated nasal spray influenza vaccine (LAIV4), with no preference expressed for any one vaccine over another (CDC, 2019l).
The nasal spray is approved for use in non-pregnant individuals, 2 years through 49 years of age. People with some medical conditions should not receive the nasal spray flu vaccine. For more information, click here.
Immunity
Immunity following administration of inactivated influenza vaccine is less than 1 year, due to waning of vaccine-induced antibodies and antigenic drift of circulating influenza viruses. Influenza vaccine efficacy varies by the similarity of the vaccine strain to circulating strains and the age and health of the recipient.
CDC conducts studies each year to determine how well the influenza vaccine protects against flu illness. While vaccine effectiveness (VE) can vary, recent studies show that flu vaccination reduces the risk of flu illness by between 40% and 60% among the overall population during seasons when most circulating flu viruses are well-matched to the flu vaccine. In general, current flu vaccines tend to work better against influenza B and influenza A(H1N1) viruses and offer lower protection against influenza A(H3N2) viruses (CDC, 2018f).
Antibody against one influenza virus type or subtype confers limited or no protection against another type or subtype. Frequent emergence of antigenic variants through antigenic drift is the virologic basis for seasonal epidemics and necessitates consideration for adjustment of vaccine viruses each season (Grohskopf et al., 2018).
Pregnant Women and Neonates
Both ACIP and the American College of Obstetricians and Gynecologists (ACOG) recommend that women who are or will be pregnant during influenza season receive an inactivated influenza vaccine as soon as it is available. Because pregnant women are at high risk of serious flu complications, CDC recommends influenza vaccination during any trimester of pregnancy (CDC, 2019c).
Influenza is more likely to cause severe illness in pregnant women than in women who are not pregnant, particularly during the second and third trimesters. Changes in the immune system, heart, and lungs during pregnancy make pregnant women (and women up to 2 weeks postpartum) more prone to severe illness from flu, including illness resulting in hospitalization. Flu also may be harmful for a pregnant woman’s developing baby. A common flu symptom is fever, which may be associated with neural tube defects and other adverse outcomes for a developing baby (CDC, 2019c).
Numerous studies have shown that flu vaccination protects pregnant women during and after pregnancy and also protects babies from flu infection for several months after birth, before the child is old enough to be vaccinated (the mother passes antibodies on to the developing baby during her pregnancy) (CDC, 2019c).
Millions of flu vaccines have been given for decades, including to pregnant women. Numerous studies, including clinical trials and observational studies, and data from vaccine safety monitoring systems have demonstrated consistently the safety of influenza vaccination during pregnancy (CDC, 2019c).
Immunocompromised People
ACIP recommends that persons with immunocompromising conditions (including but not limited to persons with congenital and acquired immunodeficiency states, persons who are immunocompromised due to medications, and persons with anatomic and functional asplenia) should receive an age-appropriate IIV or RIV4. ACIP recommends that LAIV4 not be used for these groups because of the uncertain but biologically plausible risk for disease attributable to the live vaccine virus (Grohskopf et al., 2020).
Immunocompromised states comprise a wide range of conditions with varying risks for severe infections. In many instances, limited data are available regarding the use of influenza vaccines in the setting of specific immunocompromised states. Timing of vaccination might be a consideration (e.g., vaccinating during some period either before or after an immunocompromising intervention). The Infectious Diseases Society of America (IDSA) has published detailed guidance for the selection and timing of vaccines for persons with specific immunocompromising conditions. Immune response to influenza vaccines might be blunted in persons with some conditions, such as persons with congenital immune deficiencies, and persons receiving cancer chemotherapy or immunosuppressive medications (Grohskopf et al., 2020).
High-Risk Households
Efforts should be made to vaccinate household and other close contacts of high-risk people. These include healthcare personnel, employees of long-term care facilities, and household contacts of high-risk people. These individuals may be younger and healthier and more likely to be protected from illness than are older adults. All healthcare providers should receive annual inactivated influenza vaccine (CDC, 2019, 2015PB).
Groups to be targeted include physicians, nurses, and other personnel in hospitals and outpatient settings who have contact with high-risk patients in all age groups, and providers of home care to high-risk people (CDC, 2019, 2015PB).
Older Adults
Because of the vulnerability of older adults to severe influenza illness, hospitalization, and death, efficacy and effectiveness of influenza vaccines among older adults is an area of active research. Recent comparative studies of vaccine efficacy/effectiveness against laboratory-confirmed influenza outcomes among older adults have focused on HD-IIV3 (Fluzone High-Dose), RIV4 (Flublok Quadrivalent), and aIIV3 (Fluad). Each of these three vaccines has been studied in comparison to a standard dose, unadjuvanted IIV (Grohskopf et al., 2020).
A meta-analysis reported that HD-IIV3 (Fluzone High-Dose) provided better protection than SD-IIV3 (Fluzone Standard-Dose) against influenza related illness; all-cause hospitalizations; and hospitalizations due to influenza, pneumonia, and cardiorespiratory events. For the 2020–21 season, HD-IIV3 is expected to be replaced by Fluzone High-Dose Quadrivalent (HD-IIV4) (Grohskopf et al., 2020).
Although HD-IIV3 (Fluzone High-Dose) has been the most extensively studied, and evidence has accumulated for its superior efficacy and effectiveness compared with SD-IIV3 (Fluzone Standard-Dose) in this population, no preference is expressed for any one vaccine type. Vaccination should not be delayed if a specific product is not readily available. For persons aged ≥65 years, any age-appropriate IIV formulation (standard-dose or high-dose, trivalent or quadrivalent, unadjuvanted or adjuvanted) or RIV4 are acceptable options (Grohskopf et al., 2020).
