Recall that in James Parkinson’s 1817 Essays on the Shaking Palsy he made the following observation:
So slight and nearly imperceptible are the first inroads of this malady, and so extremely slow is its progress, that it rarely happens that the patient can form any recollection of the precise period of its commencement.
New research is shedding light on what James Parkinson’s called “the precise period of its commencement.” In this emerging field of study, the classic clinical symptoms of PD are increasingly thought to be preceded by a wide variety of symptoms that may manifest years before the onset of motor symptoms and may serve as possible “premotor” or “preclinical” biomarkers for PD.
Biomarkers for PD
Biomarkers are biologic indicators of disease or therapeutic effects that can be measured by in vivo biomedical or molecular imaging as well as laboratory methods (Clarke, 2006). Biomarkers can include changes in body chemistry or physiology or changes in genes and how they are regulated. Even subtle changes in a persons behavior may be a biomarker. Currently, there are no proven biomarkers for Parkinson’s disease. Biomarkers are used, however, in the successful detection of many other diseases (NIH, 2013b).
Finding a biomarker that aids in the early detection of PD may provide information about the cause of PD and its progression, and lead to treatments that delay the progression of the disease. As with any biomarker, one for PD must be specific for Parkinson’s disease and sensitive to every person who has the disease. A good PD biomarker should identify someone who is beginning to undergo metabolic changes associated with PD before substantial injury has occurred. It should measure disease activity and progression and assist in determining the benefit of treatments and neuroprotective therapies (Christine, 2011a).
The range of potential biomarkers for Parkinson’s is vast, and there have been some promising leads. For example, researchers are investigating the use of noninvasive imaging to detect changes in brain function or brain biochemistry. This is a promising area for research because several studies have tentatively linked PD with changes in proteins or other molecules in blood, urine, or the cerebrospinal fluid (NIH, 2013b).
There is a pressing need for an accurate, relatively noninvasive, and affordable PD diagnostic test or biomarker. This is particularly true given widespread recognition that early detection and early treatment helps to slow the progression of the disease, minimize symptoms, and improve the patient’s overall quality of life. Currently, there is no one imaging technique or test that can provide a conclusive primary diagnosis of PD. There are also no laboratory tests utilizing blood, cerebrospinal fluid, or urine samples that have proven to be effective in primary diagnosis or confirmation of PD (Han et al., 2012).
Imaging Biomarkers
Functional imaging techniques such as positron emission tomography (PET) and single photon computed emission tomography (SPECT) can support the diagnosis of PD but are usually limited to a research setting. Computed tomography (CT) and magnetic resonance imaging (MRI) brain scans of people with PD usually appear normal.
Current imaging techniques are used mostly to exclude other diseases, such as basal ganglia tumors, vascular pathology, and hydrocephalus. A specific technique, diffusion MRI, has been reported to be useful at discriminating between typical and atypical Parkinson’s, although its exact diagnostic value is still under investigation.
Two widely-used imaging techniques, fluorodopa PET and DaTSCAN focus on dopamine, using radiotracers to measure dopamine function in the basal ganglia. Unfortunately, dopamine biomarkers only detect changes in dopamine after the disease is well established. A substantial fraction of patients with early idiopathic Parkinson’s disease have normal scans, and the costs and use of intravenous radioactive tracers are seen as important disadvantages of this technique (Bouwmans et al., 2013). In addition to these two types of scans, transcranial sonography is showing promise as a diagnostic tool.
Fluorodopa PET Scan
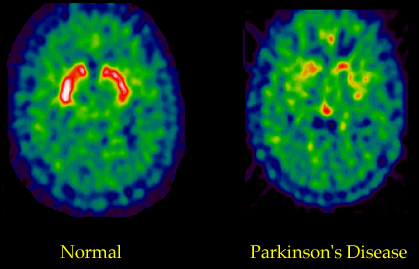
Fluorodopa (FDOPA) is a fluorinated form of L-dopa that is synthesized for use as a radiotracer in PET scans. Current studies employing the use of FDOPA PET scanning have focused on analyzing the efficiency of neurons in the striatum that utilize dopamine. This test is useful in distinguishing PD from other types of neurodegeneration.
The pictures below are examples of a PET scan that has utilized fluorodopa as a radiotracer. The bright orange areas in the scan on the left show a robust uptake of fluorodopa in the striatum—indicating normal dopamine function. The image on the right shows much less uptake of the fluorodopa, indicating a significant loss of dopamine receptors in a person with PD.
Fluorodopa PET Scan
DaTSCAN
DaT (dopamine transporter) imaging scans look at the function of presynaptic dopamine transporters. The DaTSCAN technique has the potential to predict the course of the disease by measuring the number of dopamine transporters when compared to normal levels at an early point of PD. This may be predictive of how advanced the disease will be in five years. Generally a pattern of reduced dopaminergic activity in the basal ganglia can aid in diagnosing PD (Cummings, 2011).
One-Sided Deficit in Parkinson’s Disease
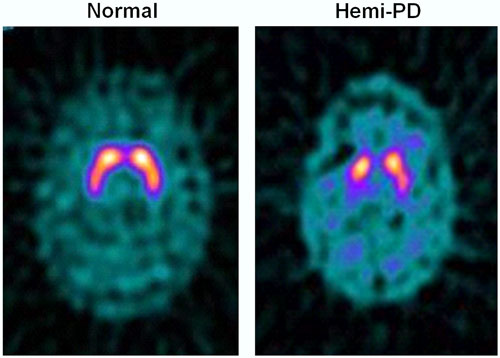
123I-FP-CIT SPECT images of healthy volunteer and patient with early hemi-PD. PD patient shows asymmetric bilateral loss of putamen DAT binding.
Transcranial Sonography
The search for a cheap and patient-friendly technique to diagnose PD has continued, and over the past ten years transcranial sonography of the substantia nigra has emerged as a promising tool. Numerous ultrasound studies have found that a significant percentage of patients with idiopathic PD have a typical enlarged area in the substantia nigra, which is thought to be associated with increased iron concentrations (Boewmans, 2013).
Among the new techniques, transcranial B-mode duplex sonography has been drawing a lot of attention as an easily accessible and inexpensive imaging method. Transcranial sonography shares some features of the functional imaging methods, which is thought to detect very early dysfunction of the nigrostriatal pathways. This probably demonstrates an increased vulnerability of the extrapyramidal system, or even an increased risk of PD development (Laučkaitė, et al., 2012).
Hyperechogenicity* of the substantia nigra, both in echo intensity and size of area, has been repeatedly reported as a characteristic transcranial sonography finding in PD patients. It can be also detected in some other neurodegenerative and even in non-neurodegenerative disorders, or just related to aging. Therefore, the issue regarding sensitivity and specificity of transcranial sonography is still disputable. While many studies have estimated specificity of transcranial sonography comparing PD patients to healthy controls or essential tremor group, to date very few case control studies involved patients with atypical Parkinson’s syndromes, neurodegenerative hereditary, or secondary Parkinson’s (Laučkaitė, et al., 2012).
*Hyperechogenicity is an increased response (echo) during the ultrasound examination of an organ, usually as a result of fatty deposits.
Transcranial sonography has also shown brainstem abnormalities in approximately 90% of patients with PD but the size of the abnormality does not correlate with disease severity and does not change over five years despite progression of symptoms (Christine, 2011a).
Genetic Biomarkers
Genetic testing can identify a trait or susceptibility for Parkinson’s disease but it is not used to determine the presence or progression of the disease. The presence of a certain gene does not definitively indicate that PD will develop. Genetic tests can be used to test for the presence of certain gene mutations but cannot be used to make a diagnosis of PD because the presence of the gene is not definitive. Recessive gene testing provides information but is not ideal for biomarkers because the onset and progression of the disease is so slow (Christine, 2011a).
Alpha-Synuclein Biomarkers
The alpha-synuclein protein is a major component of Lewy bodies and its accumulation likely precedes a diagnosis of PD by many years. The ability to identify a biomarker for the presence of alpha-synuclein is the subject of intense research.
One technique uses a molecular probe based on the metallic element ruthenium to look inside living cells in tissue culture and see the insoluble fibrillar deposits associated with Parkinson’s disease. In tests using live neuroglioma cells, the color probe binds to misfolded alpha-synuclein proteins that clump together and form Lewy bodies. The ruthenium complex lights up as a red color when triggered by a laser, but only when it was bound to the fibril, allowing alpha-synuclein aggregation to be tracked using photoluminescence spectroscopy (Cook, 2012).
It is hoped that a molecular detector can be used to monitor the formation of aggregates inside live cells while screening for drugs that break up fibrils or prevent them from forming. The ruthenium complex itself has no therapeutic benefit at this time (Cook, 2012).
Abnormal alpha-synuclein aggregation may begin in the peripheral nervous system, possibly in the nerves of the gastrointestinal submucosa many years before motor symptoms appear. In one study, colon tissue extracted during a colonoscopy was analyzed in patients in the early stages of PD but who had not been treated for PD. Tissue samples showed that 9 out of 10 had alpha-synuclein inclusions in the tissue (Christine, 2011a).
A vaccine that primes the human immune system to destroy alpha-synuclein has entered clinical trials in humans. Considered a disease-modification strategy (as opposed to symptomatic treatment), the two-year study involves four injections intended to stimulate an immune system response to alpha-synuclein. Removing alpha-synuclein may have the potential to modify the course of the disease.
Screening for Biomarkers
Screening for biomarkers employs techniques that look for patterns of variation in genes, proteins, and small molecules using a biologic sample such as saliva, blood, urine, or spinal fluid. The Michael J. Fox Foundation is using these techniques in an ongoing study of biomarkers called the Parkinson’s Progression Markers Initiative (PPMI). They are looking at movement, cognitive, and brain biomarkers in addition to blood, urine, DNA, and spinal fluid sampling in 400 newly diagnosed PD patients over a 3- to 5-year period (Christine, 2011a).
Rating Scales for PD
In clinical practice the diagnosis of idiopathic Parkinson’s disease, delineating it from the atypical parkinsonism, vascular parkinsonism, drug-induced parkinsonism, essential tremor, other neurodegenerative and movement disorders is still difficult. Especially in the early stage of these diseases, a large group of patients is erroneously diagnosed, even by experienced movement disorder specialists, when compared to postmortem findings (Bouwmans et al., 2013).
Parkinson’s disease is clinically classified according to the age of onset. If symptoms begin after age 50 it is usually referred to as late-onset disease. The condition is described as early-onset disease if signs and symptoms begin before age 50. Cases that begin before the age of 20 are sometimes referred to as juvenile-onset Parkinson’s disease. The late-onset form is the most common type of Parkinson’s disease, and the risk of developing it increases with age.
There are several rating scales used to determine the presence of Parkinson’s disease, assess its severity, and monitor its progression. One of the most commonly used is the Unified Parkinson’s Disease Rating Scale (UPDRS), which was first developed in 1987 and is used extensively throughout the world. The International Classification of Functioning, Disability, and Health (ICF) is another rating scale that looks at body structure and function, activity and participation, and environment. The Hoehn and Yahr scale, in use since it was developed in 1967, is also widely used to describe how symptoms progress. The Hoehn and Yahr Rating Scale measured progression of the disease but has been largely replaced by the UPDRS. The Schwab and England ADL scale is used to determine levels of independence.
Unified Parkinson’s Disease Rating Scale
Currently the Unified Parkinson’s Disease Rating Scale (UPDRS) and its most recent version, the MDS-UPDRS, are considered the gold standards for determining the severity and progression of Parkinson’s disease. However, the UPDRS focuses primarily on measuring impairments associated with PD, with fewer items addressing specific functional limitations or perceptions of quality of life. The MDS-UPDRS is divided into four main areas: (I) non-motor experiences of daily living, (II) motor experiences of daily living, (III) motor examination, and (IV) motor complications (Dibble et al., 2012).
The motor examination section (part III) of the UPDRS is the most widely used measure to assess motor symptoms and signs in PD; it is the only part of the UPDRS scored by the healthcare provider rather than by patient self-report. However, examining motor abnormalities may not reveal the beneficial effects of treatments that target certain motor components or enable identification of subsets of patients with different motor profiles and prognoses (Vassar et al., 2012).
Because the UPDRS is organized according to motor and non-motor aspects of PD, it has limited focus on the assessment of disability. As a result, PD is commonly understood more in terms of disease progression (ie, the predictable evolution of signs, symptoms, and impairments) rather than in terms of the potentially diverse paths through which persons with PD becomes disabled (Dibble et al., 2012).
International Classification of Functioning, Disability, and Health (ICF)
In contrast to the UPDRS, the International Classification of Functioning, Disability, and Health (ICF) was developed to provide an underlying framework for understanding the consequences of a disease from body, individual, and societal perspectives. The effects of a disease are considered across three domains of human function: (1) body structure and function, (2) activity and participation, and (3) environmental factors.
In the ICF, disability is used to denote a decrement at each level (ie, a body structure or functional impairment, an activity limitation, a participation restriction). Underscoring the value of this approach, the World Health Organization (WHO) endorsed the use of the ICF in 2001 as the international standard to describe and measure health and disability (Dibble et al., 2010).
The ICF puts the notions of “health” and “disability” in a new light. It acknowledges that every human being may experience a decrement in health and thereby some degree of disability; that is, disability is not something that only happens to the few. The ICF “mainstreams” the experience of disability and recognizes it as a universal human experience.
By shifting the focus from cause to impact, the ICF places all health conditions on an equal footing, allowing them to be compared using a common metric—the ruler of health and disability. Furthermore, ICF takes into account the social aspects of disability and does not see disability only as a medical or biologic dysfunction. By including environmental factors to provide context, the ICF examines the impact of the environment on the person’s functioning (WHO, 2013).
Body Structure and Function
Body structure, in ICF terms, is defined as an anatomical part of the body, such as organs, limbs and their components, while body function is defined as the physiologic function of body systems. Applied to PD, motor signs such as bradykinesia, tremor, and rigidity represent impairments in body structure and body function (Dibble et al., 2010).
Impairments in body structure and body function are rated using a scale, which describes the extent of impairment from no impairment (no problems) to complete impairment (problem is present more than 95% of the time, with an intensity that is totally disrupting the persons day-to-day life and happened every day over the last 30 days).
Body structure includes:
- The nervous system
- Eyes, ears, and related structures
- Structures involved with voice and speech
- Structures of the cardiovascular, immunologic, and respiratory systems
- Structures related to the digestive, metabolic, and endocrine systems
- The genitourinary and reproductive systems
- Structures related to movement
- Skin and related structures
- Any other body structures
Body function includes:
- Mental functions
- Sensory functions and pain
- Voice and speech functions
- Functions of the cardiovascular, hematologic, immunologic, and respiratory systems
- Functions of the digestive, metabolic, and endocrine systems
- Genitourinary and reproductive functions
- Neuromusculoskeletal and movement-related functions
- Functions of the skin and related structures
- Any other body functions
Activity and Participation
Activity is defined as the execution of a task or action by an individual. Activity limitations are the difficulties an individual may have in executing such tasks. Activity limitations common in PD are those affecting gait, balance, dressing, bathing, and other activities of daily living (Dibble et al., 2010).
Participation is defined as the involvement in a life situation. Participation restrictions are the problems an individual may experience in involvement in life situations. Participation restrictions may affect leisure activities, work, and social aspects of life in both the household and community settings (Dibble et al., 2010).
Activity limitation and participation restrictions are rated using a scale, which describes the extent of participation restriction and the extent of activity limitation from no difficulty (no problem) to complete difficulty (problem that is present more than 95% of the time, with an intensity that is totally disrupting the person’s day-to-day life and which happened every day over the last 30 days).
Activity and participation domains include:
- Learning and applying knowledge
- General tasks and demands
- Communication
- Mobility
- Self-care
- Domestic life
- Interpersonal interactions and relationships
- Major life areas
- Community, social, and civic life
- Any other activity and participation
Environmental Factors
Environmental include the physical, social, and attitudinal environment in which people live and conduct their lives. This section of the ICF uses a scale to rate barriers (no barriers to complete barriers) and facilitators (no facilitator to complete facilitator).
Environmental factors include:
- Products and technology
- Natural environment and human-made changes to the environment
- Support and relationships
- Attitudes
- Services, systems, and policies
- Any other environmental factors
For a detailed and current version of the ICF at the World Health Organization’s website, click here.
Hoehn and Yahr Staging Scale
The Hoehn and Yahr scale describes how symptoms progress in PD. It has been widely used because it is simple and identifies patterns of progressive motor impairment. It does not provide information about non-motor aspects of PD. It was first published in 1967 and has largely been replaced by the more thorough UPDRS scale. A modified version of the original scale is available (shown here).
|
Modified Version of Original Hoehn and Yahr Staging Scale |
|
|---|---|
|
Stage |
Description |
|
1 |
Unilateral involvement only. |
|
1.5 |
Unilateral and axial involvement |
|
2 |
Bilateral involvement without impairment of balance. |
|
2.5 |
Mild bilateral disease with recovery on pull test. |
|
3 |
Mild to moderate bilateral disease; some postural instability; physically independent |
|
4 |
Severe disability; still able to walk and stand unassisted. |
|
5 |
Confinement to bed or wheelchair unless aided |
Schwab and England ADL Scale
The Schwab and England Activities of Daily Living scale assesses daily activities in terms of speed and independence. It uses a scale divided into 10% increments starting at 100% (complete independence in all activities without slowness, difficulty, or impairment) and moving to 0% (vegetative functions such as swallowing, bladder and bowel are not functioning; bedridden).
