Galen of Pergamon
(a.d. 129–c. 200)

Also known as Aelius Galenus, roman physician from Pergamon, Turkey, the most famous medical researcher of classical antiquity. Lithograph by Pierre Roche Vigneron.
The symptoms associated with Parkinson’s disease have been observed throughout human history. Early descriptions of people with symptoms date back thousands of years. An Ayurvedic medical treatise from India of the tenth century B.C.E. describes a disease that evolves with tremor, lack of movement, drooling, and other symptoms characteristic of PD.
In A.D. 175 the medical researcher Galen described the symptoms now associated with PD: tremors while at rest, postural stooping, and paralysis. Others through the centuries described one or several of the characteristic symptoms but without clear understanding of the cause or the progression of the disease.
Early treatments for PD involved plants of the mucuna family of tropical vines. Mucana pruriens seeds, also known as the tropical legume “velvet bean” or “cow itch,” are rich in levodopa, a direct molecular precursor of the neurotransmitter dopamine. The seeds are native to Africa, India, and the Caribbean, and have long been used in traditional Ayurvedic Indian medicine for the treatment of Parkinson’s and other diseases.

Left: The mucuna pruriens, a hand-colored engraving after a drawing by Miss S.A. Drake, from Vol. 24 of the Botanical Register (1838), edited by John Lindley. Public Domain. Right: Mucuna pruriens seeds of two different colors, each about the size of a chicken egg. Source: USDA, 2012.
In Central America and Brazil, velvet beans have been roasted and ground for decades to make a coffee substitute with the common name of nescafé. Single portions, approximately 1 ounce of the seeds, have been shown to be as effective as single doses of modern medicines in the treatment of Parkinson’s disease, but long-term efficacy and tolerability have not been determined.
Mucuna seeds also contain several other potent neuroactive and psychoactive compounds. These include serotonin, 5-hydroxytryptophan (5-HTP), nicotine, dimethyltryptamine (DMT), bufotenin, and 5-MeO-DMT, the last three being powerful psychedelic tryptamines. Extracts can exhibit strong psychedelic effects, and are reportedly used in the South American ayahuasca (medicinal tea) preparations.
Early Clinical Descriptions
Although the physical symptoms and rudimentary medical descriptions were around for many centuries, one of the earliest clinical descriptions identifying PD as a neurologic syndrome is found in An Essay on the Shaking Palsy, published in 1817 by the London physician James Parkinson. He described six individuals, each with similar clinical features:
James Parkinson (1755–1824)

Source: Courtesy of allaboutParkinsons.com
So slight and nearly imperceptible are the first inroads of this malady, and so extremely slow is its progress, that it rarely happens that the patient can form any recollection of the precise period of its commencement. The first symptoms perceived are a slight sense of weakness with a proneness to trembling in some particular part; sometimes in the head, but most commonly in one of the hands and arms.
These symptoms gradually increase in the part first affected; and at an uncertain period, but seldom in less than twelve months or more, the morbid influence is felt in some other part. After a few more months the patient is found to be less strict than usual in preserving the upright posture: this being most observable whilst walking, but sometimes whilst sitting or standing. (Parkinson, 1817)
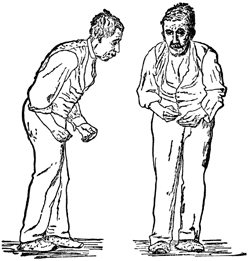
Front and side views of a man with a festinating gait characteristic of Parkinson’s disease. Drawing after St. Leger, first published in Wm. Richard Gowers’ Diseases of the Nervous System, in London, 1886. Source: Wikipedia.
With the publication of his essay, James Parkinson provided the first formal clinical description the disease, which focused on tremor, weakness, rigidity, and postural and gait changes. Parkinson wrote that patients with the shaking palsy exhibited:
- Involuntary, tremulous motion with lessened voluntary muscle power, in parts, not in action, and even supported
- A propensity to bend the trunk forwards, and to pass from walking to a running pace
Building on Parkinson’s work, in 1871 Theodor Meynart, a German-Austrian neuropathologist, recognized that a part of the brain called the basal ganglia was involved with abnormal movements. Shortly after Meynart’s discovery and more than seventy years after Parkinson published his essay, Jean-Martin Charcot, in his Clinical Lectures on Diseases of the Nervous System (Charcot, 1889), refined Parkinson’s clinical description of shaking palsy (or “paralysis agitans”) by adding bradykinesia or slowness of movement as a defining feature of the disease. Charcot wrote:
Jean-Martin Charcot (1825–1893)

Source: Wikimedia Commons
Long before rigidity actually develops, patients have significant difficulty performing ordinary activities: this problem relates to another cause. In some of the various patients I showed you, you can easily recognize how difficult it is for them to do things even though rigidity or tremor is not the limiting feature. Instead, even a cursory exam demonstrates that their problem relates more to slowness in execution of movement rather than to real weakness.
In spite of tremor, a patient is still able to do most things, but he performs them with remarkable slowness. Between the thought and the action there is a considerable time lapse. One would think neural activity can only be effected after remarkable effort.
The addition of bradykinesia as one of the cardinal features led Charcot to suggest a new name honoring James Parkinson’s early work. He was the first to call it “Parkinson’s disease,” rather than shaking palsy or paralysis agitans, arguing that tremor was not always present, nor was paralysis.
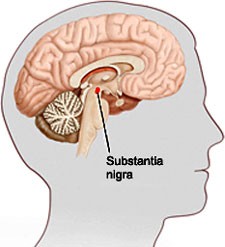
An illustration showing the location of the substantia nigra. Source: veteranshealthlibrary.org.
Just a few years later, in 1895, Edouard Brissaud, a student of Charcot, became the first researcher to suggest that damage to a part of the midbrain called the substantia nigra—so named because it appears darker than neighboring areas—was the cause of Parkinson’s disease. Then, in 1912, in a breakthrough that was to have lasting implications, Frederic Lewy identified what he called “spherical neuronal inclusions” on a pathology slide from the brain of a deceased Parkinson’s patient. Seven years later Konstantin Tretiakoff, a Russian neuropathologist, named the inclusions corps de Lewy or Lewy bodies (Kasuga et al., 2012). Further research confirmed Brissaud’s observations that the substantia nigra was the main cerebral structure affected in people with Parkinson’s disease, but this was not widely accepted until confirmation by further studies published in 1938.
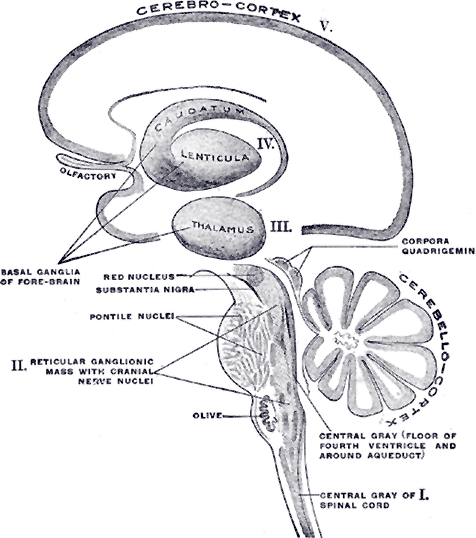
Schematic representation of the chief ganglionic categories by Henry Gray. Note the location of the basal ganglia above the thalamus and the substantia nigra at the top of the brainstem just below the thalamus. From Gray’s Anatomy, 1918. Source: courtesy bartleby.com.
The Poskanzer and Schwab Hypothesis
[This section taken largely from Estupinan et al., 2013.]
In the long history of research into the cause of Parkinson’s disease, one important hypothesis should be noted. In 1956 two Harvard-based neurologists, David C. Poskanzer and Robert S. Schwab, put forth a bold hypothesis that Parkinson’s disease was caused by influenza and that the disease would die out by 1980. Their hypothesis linked influenza infection during the 1918 influenza pandemic with “Parkinsonism.”
According to Poskanzer, one of the original inspirations for his hypothesis was derived from a 1956 study that reported Parkinson’s disease prevalence shifting toward an older age group when compared to a previous seven-year period. Sixty percent of the older patients interviewed—all but one born before 1927—recollected a history of influenza infection. Poskanzer was struck by the possibility that a viral infection could be the underlying cause of Parkinson’s disease.
In 1961, at the Eighty-sixth American Neurological Association meeting held in Atlantic City, New Jersey, Poskanzer and Schwab presented a paper, “Studies in the Epidemiology of Parkinson’s Disease Predicting Its Disappearance as a Major Clinical Entity by 1980.” Their paper formally presented the hypothesis that Parkinson’s disease was caused by previous influenza viral infection. The paper and the hypothesis were both received with skepticism. The hypothesis received public attention in a 1962 New York Times article that linked “palsy to virus” and the prediction that some researchers believed “Parkinsonism” would die out in 20 to 40 years.
Bulletin
October 19, 1962 (The New York Times). New Theory Links Palsy to a Virus; Two Researchers Believe Parkinsonism May Vanish in 20 to 40 Years (By John A. Osmundsen, Special to The New York Times)
In 1963 Poskanzer and Schwab published more studies postulating a direct link between viral exposure and Parkinson’s disease. The two neurologists reported that the cohorts of Parkinson’s patients exposed to influenza during two successive pandemics (1920–1924 and 1955–1959) had the greatest incidence of Parkinson’s disease. As a result of these collective observations, the two neurologists asserted that the incidence of Parkinson’s disease would dramatically tail off and perhaps even disappear with the death of all influenza sufferers.
Poskanzer was so confident of his theory that, in a 1974 Time magazine article entitled “The Parkinson’s Puzzle,” he famously challenged: “I offer a bottle of scotch to any doctor in the U.S. who can send me a report of a clearly diagnosed case of Parkinson’s in a patient born since 1931. So far it’s cost me 14 bottles—just 14 of these younger patients identified since 1961.”
Although Parkinson’s disease is currently defined as an idiopathic disease—no specific cause has been identified—influenza may provide the first “hit” that leads to the later development of Parkinson’s disease, suggesting a possible mechanism for viral infection in disease manifestation. More important, despite discounting Poskanzer and Schwab’s initial hypothesis, the association between virus exposure and Parkinson’s disease is still being actively studied (Estupinan et al., 2013).
The Development of Levodopa (L-dopa)
Arvid Carlsson
(b. 1923)

Source: nobelprize.org
In the first half of the twentieth century, many medical and surgical strategies were used in an attempt to alleviate the tremors and other symptoms associated with Parkinson’s. Surgery—such as ablating part of the basal ganglia to reduce tremor—was first tried in 1939 and was improved over the following twenty years. Anesthetic anticholinergic agents (used to reduce nerve impulses to muscles) were the only available drug treatments available for tremors until the discovery and widespread use of levodopa.
Dopamine was first identified as an independent neurotransmitter in 1957, a discovery that had a profound impact on the field of neuroscience. The work, by Arvid Carlsson and his colleagues in Sweden, quickly led to the discovery of the first dopamine receptor. The understanding that Parkinson’s disease was associated with the depletion of dopamine led to the development of the first drug treatment for Parkinson’s disease: L-3,4-dihydroxyphenylalanine (L-dopa, levodopa), which is still used today (Meiser et al., 2013). Carlsson demonstrated that administering L-dopa to animals with parkinsonian symptoms reduced the intensity of their symptoms.
Oliver Sacks
(1933–2015)

Neurologist and writer Oliver Sacks at the 2009 Brooklyn Book Festival. Copyright © Luigi Novi/Wikimedia Commons.
L-dopa entered clinical testing and use in 1968 after a large study reported that people with Parkinson’s disease showed significant improvement following treatment with levodopa. Levodopa brought about a true paradigm shift in the management and understanding of PD. Carlsson was awarded the Nobel Prize in 2000 for his work with dopamine.
Levodopa was famously used by neurologist Oliver Sacks in his patients with encephalitis lethargica (detailed in his 1973 book and later movie, Awakenings). Sacks’ patients were frozen motionless with post-encephalitic parkinsonism, a form of Parkinson’s that causes degeneration of the nerve cells in the substantia nigra and is thought to be viral in origin. Unfortunately, the post encephalitic patients lost the benefits of L-dopa treatment far faster than do patients with Parkinson’s disease.
Alan: Living with Parkinson’s
In late 1995 I learned that I might have Parkinson’s disease.
Several years earlier, I had noticed some changes in my body when playing first base in the men’s softball league. The last game I played, I missed three throws from the shortstop. I didn’t just drop the ball—I never even got a glove on it. This hesitation in reaching for the ball may have been my first indication of Parkinson’s.
The next thing I noticed is that suddenly I began having extra a’s in my news copy when I typed. Then one day I tripped going up the stairs to the restroom at the paper. The tripping became more frequent and began happening when I was just walking across the carpet.
It was at the Chamber’s summer mixer in 1995 that I first heard the “P” word. I was talking to a realtor who had previously been a nurse. I told her that I was having some shaking in my left arm. I thought it had something to do with a shoulder injury sustained several years earlier while playing softball.
“Have you ever been checked for Parkinson’s?” she asked.
“Isn’t that an older person’s disease?” I shrugged. I was 47 years old.
Several weeks later, a friend had emergency surgery to remove a brain tumor. After she recovered, I asked her what symptoms had preceded the seizure that sent her to the hospital. She said she had had some minor headaches, but two weeks before the seizure she was putting her makeup on and started drooling from the left side of her mouth. I told her I was experiencing the same things.
“Go get it checked,” she echoed the earlier advice.
I made an appointment at the local health center. From the exam my doctor suspected something was going on so he scheduled me for an MRI. I went back to see him several days after the test.
“We scanned your brain and didn’t find anything,” said the doc.
“Maybe that’s why I’m having memory loss—there isn’t any brain up there,” I quipped.
“No this is a good thing, there are no tumors. But I’m going to send you to a neurologist.”
This neurologist turned out to be very thorough, and knowledgeable about Parkinson’s.
He tried me on Sinemet and the symptoms went away.
“This is a good thing, right?”
“I’d say you are in the early stages of Parkinson’s.”
By this time it was February 1996.
