Because drug abuse and addiction have so many dimensions and disrupt so many aspects of a person’s life, treatment is not simple. Most people cannot simply stop using drugs for a few days and be cured.
Effective treatment incorporates many components, each directed to a particular aspect of the illness and its consequences. Patients usually require long-term or repeated episodes of care for sustained abstinence and recovery.
The goal of addiction treatment is to help a person stop using drugs, maintain a drug-free lifestyle, and achieve productive functioning in the family, at work, and in society. To be successful, treatment must be readily available and address the multiple needs of the individual, not just his or her drug abuse (NIDA, 2018, January).
Medications are an important element of treatment for many patients, especially when combined with counseling and behavioral therapies. Medications may be taken for varying lengths of time, including lifelong treatment (NIDA, 2018, January).
Medically assisted withdrawal (detoxification) is only the first stage of addiction treatment and by itself does little to change long-term drug abuse. Detoxification is not in itself "treatment," but only the first step in the process. Patients who do not receive any further treatment after detoxification usually resume their drug use (NIDA, 2019, January).
Because drug abuse and addiction often co-occur with other mental illnesses, patients presenting with one condition should be assessed for the other. If these problems co-occur, treatment needs to address both, including the use of appropriate medications (NIDA, 2018, January).
Drug use during treatment must be monitored continuously, as lapses during treatment do occur. Treatment programs should test patients for the presence of HIV/AIDS, hepatitis B and C, tuberculosis, and other infectious diseases, as well as provide targeted risk-reduction counseling (NIDA, 2018, January).
Unfortunately, only a minority of people with OUDs receive treatment of any kind, even after a nonfatal overdose. This represents a missed public health opportunity, given the well established effectiveness of opioid agonist treatment in reducing mortality (Tsai, et al., 2019).
As a result of the Affordable Care Act, the number of Rhode Islanders without health insurance declined by more than half between 2013 and 2016. Most of that decline was accounted for by the Medicaid expansion, which brought in nearly 90,000 new enrollees. These enrollees gained access to generous benefits for mental health and substance abuse treatment services, including full coverage for methadone treatment in specialized opioid treatment programs (OTPs) and full coverage of buprenorphine prescriptions (Burke & Sullivan, 2020).
Overview of Medications for OUD
Ongoing outpatient medication treatment for opioid use disorder is linked to better retention and outcomes than treatment without medication. Although some people stop using opioids on their own and others recover through support groups or specialty outpatient or residential treatment with or without medication, FDA-approved medication should be considered and offered to patients with OUD as part of their treatment (SAMHSA, 2020, May).
Methadone (Dolophine, Methadose), buprenorphine (Suboxone, Subutex, Probuphine, Sublocade), and naltrexone (Vivitrol) are approved by the FDA for treatment of opioid addiction. Acting on the same targets in the brain as heroin and morphine, methadone and buprenorphine suppress withdrawal symptoms and relieve cravings. Naltrexone blocks the effects of opioids at their receptor sites in the brain and should be used only in patients who have already been detoxified (NIDA, 2019, January).
Only physicians, nurse practitioners, physician assistants, and—until October 1, 2023—clinical nurse specialists, certified registered nurse anesthetists, and certified nurse midwives can prescribe buprenorphine for opioid use disorder. They must get a federal waiver to do so (SAMHSA, 2020, May).
Only federally certified, accredited opioid treatment programs (OTPs) can dispense methadone to treat OUD. Opioid treatment programs can administer and dispense buprenorphine without a federal waiver. Any prescriber can offer naltrexone, which is carried by many EMTs who are first responders (SAMHSA, 2020, May).
Did You Know?
In Rhode Island, Medicaid expansion brought in nearly 90,000 new enrollees, who gained full coverage for methadone treatment and full coverage of buprenorphine prescriptions (Burke & Sullivan, 2020).
Opioid Agonist Treatment
Opioid agonist treatment (OAT)—consisting of daily use of methadone or buprenorphine after an initial period of detoxification from other opioids—has been found to be most effective for achieving long-term abstinence from opioids of abuse. The decision of whether to take methadone, buprenorphine, or naltrexone should be specific to each patient and should consider the risks of side effects and interactions with other medications (Burke & Sullivan, 2020).
The science that demonstrates the effectiveness of these medications for opioid use disorder is strong. For example, methadone, extended-release injectable naltrexone (XR-NTX), and buprenorphine were each found to be more effective in reducing illicit opioid use than no medication in randomized clinical trials. Methadone and buprenorphine treatment have also been associated with reduced risk of overdose death (SAMHSA, 2020, May).
Once treatment is initiated, both a buprenorphine/naloxone combination and an extended-release naltrexone formulation are similarly effective in treating opioid addiction. Because full detoxification is necessary for treatment with naloxone, initiating treatment among active users is difficult, but once detoxification is complete both medications have similar effectiveness (NIDA, 2019, January).
Opioid agonist treatment with long-acting oral medications is widely used in Western countries. Most patients receiving OAT will stop or reduce their use of street opioids and may improve their physical and mental health and social connections. Another effective alternative treatment available in some European and Canadian settings, is injectable OAT (iOAT) with either diacetylmorphine (heroin) or hydromorphone (Oviedo-Joekes et al., 2021).
The delivery of injectable OAT comes with more restrictive regulatory limits compared to oral OAT. A main premise of injectable OAT is that the medications are dispensed and self-administered by injection under direct observation, for the safety of the patient and the community. When the medications are taken onsite, patients can be monitored for signs of intoxication before the injection or after, for signs of over-sedation or respiratory depression. If an overdose occurs after the injection of the medication, immediate onsite treatment is available, ensuring the safety of the patient (Oviedo-Joekes et al., 2021).
The risk for the community stems from the possibility of diversion of the patients’ medication, or its use not as prescribed, posing risk to others. Take home doses (also called carries) for injectable medications are not allowed in this framework, even if clinically advised (Oviedo-Joekes et al., 2021).
Medication Assisted Treatment (MAT)
The term medication assisted treatment refers to the use of medications in conjunction with individual and/or group counselling and other recovery support services. The World Health Organization (WHO) and the U.S. Department of Health and Human Services (DHHS) both strongly endorse the use of MAT for opioid dependence, based on its proven effectiveness in reducing abuse of opioids, risk of fatal overdose, and all-cause mortality (Burke & Sullivan, 2020).
Medication-assisted treatment provides a whole-patient approach to the treatment of substance use disorders. Medications used in MAT are approved by the Food and Drug Administration and MAT programs are tailored to meet each patient’s needs (SAMHSA, 2021).
For some people struggling with addiction, MAT can help sustain recovery. It may also prevent or reduce opioid overdose. MAT has proved to be clinically effective and to significantly reduce the need for inpatient detoxification services. These medications and therapies can also contribute to lowering a person’s risk of contracting HIV or hepatitis C by reducing the potential for relapse (SAMHSA, 2021).
The goal of MAT is full recovery, including the ability to live a self-directed life. This treatment approach has been shown to:
- Improve patient survival.
- Increase retention in treatment.
- Decrease illicit opiate use and other criminal activity among people with substance use disorders.
- Increase patients’ ability to gain and maintain employment.
- Improve birth outcomes among women who have substance use disorders and are pregnant. (SAMHSA, 2021)
Getting into approved treatment programs, where these drugs can be given and monitored closely, in combination with behavioral therapy, is often difficult, expensive, and may not be approved by some insurance companies. Many— or even most—OUD sufferers receive no relevant medications during treatment, or they receive no treatment whatsoever.
In March of 2020, in response to challenges in accessing treatment during the COVID-19 pandemic, the federal government suspended a law that required patients to have an in-person visit with a healthcare provider before they could be prescribed MAT. Through the end of the declared public health emergency, patients were temporarily able to initiate treatment over the phone without in-person or video appointments. Telehealth-delivered MAT was found to be effective in small-scale studies before the pandemic, and patients were more likely to remain in treatment uninterrupted (Burke & Sullivan, 2020).
In response to COVID-19, people with OUD are facing unique challenges, such as not being able to practice physical distancing, financial insecurity, living in shelters, or being homeless. They have other medical conditions that make them more likely to be immunocompromised and at risk of developing COVID-19 (Oviedo-Joekes et al., 2021).
Medications for Opioid Use Disorder | ||
|---|---|---|
Methadone (Dolophine, Methadose) | Synthetic opioid agonist | Eliminates withdrawal symptoms and relieves drug cravings |
Buprenorphine | Partial opioid agonist | Reduces cravings and withdrawal symptoms |
Naltrexone | Opioid antagonist | Prevents opioids from producing euphoria |
Lucemyra (Lofexidine)
Lofexidine (Lucemyra) is a non-opioid centrally acting alpha2-adrenergic receptor agonist that was first approved for the treatment of opioid withdrawal in the United Kingdom in 1992. It was originally studied for use as an antihypertensive in 1980, but research was stopped as it was found less effective for the treatment of hypertension than clonidine. Lofexidine was then repurposed for the treatment of opioid withdrawal, as it was seen to be more economical and have fewer side effects than clonidine (NLM, 2021). In 2018 the FDA approved lofexidine for use in reducing symptoms associated with opioid withdrawal in adults, whether they have been using opioids appropriately or experience opioid use disorder.
The Role of Naloxone (Narcan)
Naloxone (Narcan) is a medication designed to rapidly reverse opioid overdose. It is an opioid antagonist—meaning that it binds to opioid receptors and can reverse and block the effects of opioids. It can quickly restore normal respiration to a person whose breathing has slowed or stopped due to overdosing with heroin or prescription opioid pain medications.
Naloxone Kit
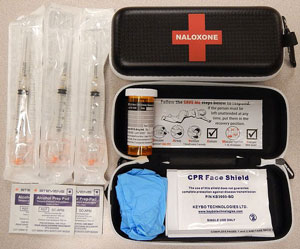
Source: James Heilman, MD, CC BY-SA 4.0, via Wikimedia Commons.
Naloxone is available as an injectable, auto injectable, or a prepackaged nasal spray. If a patient is unconscious, follow the ABCs of emergency response* such as calling 911, checking for a pulse, securing an open airway, and providing rescue breaths. Patients will often respond quickly and be confused and possibly combative.
*ABCs: Airway, Breathing, and Circulation.
Most states have passed laws to widen the availability of naloxone for family, friends, and other potential bystanders of overdose. In April 2019, the FDA approved the first generic naloxone hydrochloride nasal spray that can stop or reverse the effects of an opioid overdose. Naloxone nasal spray delivers a measured dose when used as directed. It can be used for adults or children and is easily administered by anyone, even those without medical training. The drug is sprayed into one nostril while the patient is lying on his or her back and can be repeated if necessary (FDA, 2019).
Naloxone Availability in Rhode Island
In Rhode Island, naloxone is available at local pharmacies without a prescription. The Good Samaritan law protects you from arrest for helping anyone you think is having an overdose (Prevent Overdose RI, 2020b).
Harm Reduction
Harm reduction is the use of strategies that promote safer use, managed use, abstinence, meeting people who use drugs “where they are,” and addressing conditions of use along with the use itself (NHRC, 2021). Harm reduction strategies include:
- Access to sterile injection equipment to reduce secondary transmission of HIV and hepatitis C
- Supervised consumption facilities and supervised treatment with diacetylmorphine (heroin) to reduce overdose risk
- Expansion of overdose education and naloxone distribution to reduce the case-fatality rate of opioid overdoses when they do occur (Tsai, et al., 2019)
