Although we are learning more each day about the pathophysiology of Parkinson’s disease, it is still considered largely idiopathic (of unknown cause). It likely involves the interaction of host susceptibility and environmental factors. A small percentage of cases are genetically linked and genetic factors are being intensely studied.
Physiologically, the symptoms associated with Parkinson’s disease are the result of the loss of a number of neurotransmitters, most notably dopamine. Symptoms worsen over time as more and more of the cells affected by the disease are lost. The course of the disease is highly variable, with some patients exhibiting very few symptoms as they age and others whose symptoms progress rapidly.
Parkinson’s is increasingly seen as a complex neurodegenerative disease with a sequence of progression. There is strong evidence that it first affects the dorsal motor nucleus of the vagus nerve and the olfactory bulbs and nucleus, then the locus coeruleus, and eventually the substantia nigra. Cortical areas of the brain are affected at a later stage. Damage to these various neuronal systems account for the multi-faceted pathophysiologic changes that cause impairments not just to the motor system but also to the cognitive and neuropsychological systems (Kwan & Whitehill, 2011).
The Role of Dopamine
Dopamine, like other neurotransmitters, transmits chemical messages from one nerve cell to another across the synapse, a space between the presynaptic cell and the postsynaptic receptor. Dopamine is secreted into the synapse from membrane storage vesicles in the presynaptic membrane. It crosses the synapse and binds to the postsynaptic membrane, where it activates dopamine receptors. Unused dopamine remaining in the synapse is absorbed back into the presynaptic cell; once back in the presynaptic cell, the excess dopamine is repackaged into storage vesicles and released once more into the synapse.
Within the synapse, as dopamine travels from one cell to another, it can be broken down and rendered inactive by two enzymes, MAO (monoamine oxidase) and COMT (catechol-O-methyl transferase). One therapeutic strategy introduces a MAO inhibitor into the synapse, which interrupts the action of the MAO enzyme and prevents the breakdown of dopamine. This allows more dopamine to remain in the synapse and increases the likelihood that it will bind to the postsynaptic membrane.
Chemical Synaptic Transmission
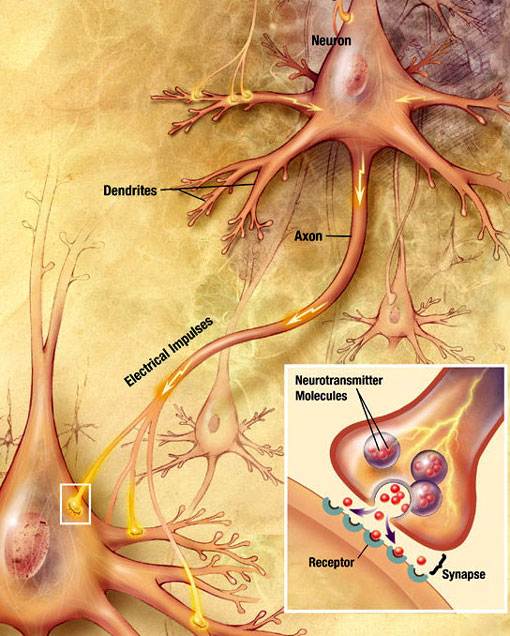
An electrochemical wave called an action potential travels along the axon of a neuron. When the action potential reaches the presynaptic terminal, it provokes the release of a small quantity of neurotransmitter molecules, which bind to chemical receptor molecules located in the membrane of the postsynaptic neuron, on the opposite side of the synaptic cleft. Source: Wikimedia Commons.
Progressive Loss of Dopamine
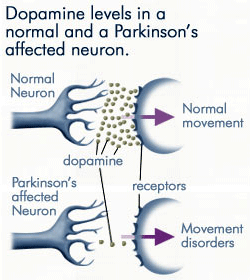
As less and less dopamine is produced by the neurons affected by Parkinson’s disease, far less dopamine is available to bind to the dopamine receptors on the post-synaptic membrane. Source: anti-agingfirewalls.com.
Although dopamine cell loss cannot be measured directly, measurements in neurologically normal people and in nonhuman primates reveal a slow progressive loss of dopamine with age. In Parkinson’s disease the loss occurs at a much greater rate and both biochemical measures and imaging studies suggest there is a significant decrease in dopamine by the time motor symptoms appear. In this view, Parkinson’s disease is an accelerated version of the cell death seen with normal aging (Cookson, 2009). This is illustrated in the graph below, which shows the decline of dopaminergic neurons during normal aging, in idiopathic PD, in PD caused by environmental or genetic factors, and in early-onset PD.
Evolution of Dopamine Depletion in Parkinson’s Disease
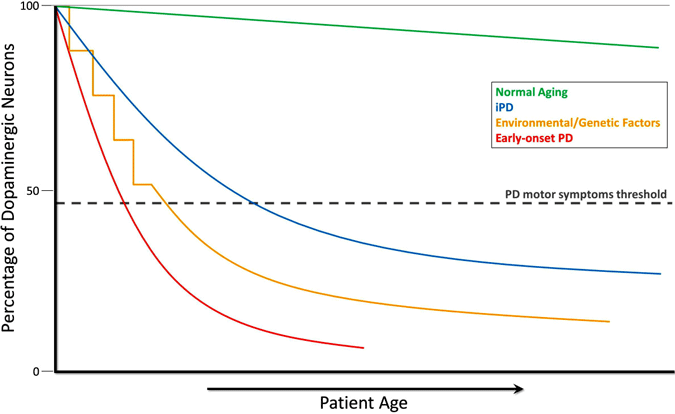
During the course of normal aging (green line), small but slow dopaminergic degeneration occurs without any motor symptoms. Idiopathic PD (IPD, blue line) is of unknown origin but is thought to develop gradually, with a slow degeneration of dopaminergic neurons leading to the classic PD motor symptoms later in life. Another model of dopamine neurodegeneration leading to PD motor symptoms involves repeated exposure to environmental toxicants over time in combination with a genetic predisposition to dopaminergic neuron loss (yellow line). Early-onset PD (red line), as caused by mutations in the PARKIN gene, involves a precipitous decline in dopaminergic neurons, and PD motor symptoms can present decades prior to those in idiopathic PD. One more scenario (not shown) of PD motor symptom development involves possible in utero environmental toxicants or genetic factors leading to an atypically low number of dopaminergic neurons at birth and increased susceptibility to PD development (Haas et al., 2012).
Degeneration of dopamine neurons is particularly evident in a part of the substantia nigra called the pars compacta. Significantly, the loss of dopamine in the pars compacta increases the overall excitatory drive in the basal ganglia,* disrupting voluntary motor control and causing the characteristic symptoms of PD. Normalization of motor function is seen initially with levodopa treatment (Gasparini et al., 2013).
*The main components of the basal ganglia are the striatum (caudate nucleus and putamen), the globus pallidus, the substantia nigra, the nucleus accumbens, and the subthalamic nucleus.
As the severity of PD increases, the depletion of dopamine leads to further changes in the basal ganglia pathways, including altered function of other basal ganglia neurotransmitters such as glutamate, GABA, and serotonin (Gasparini et al., 2013). Although there is relative vulnerability of dopamine-producing neurons in the substantia nigra, not all dopamine cells are affected in Parkinson’s disease; in some parts of the brain the dopamine-producing neurons are relatively spared (Cookson, 2009).
The Nigrostriatal Pathway
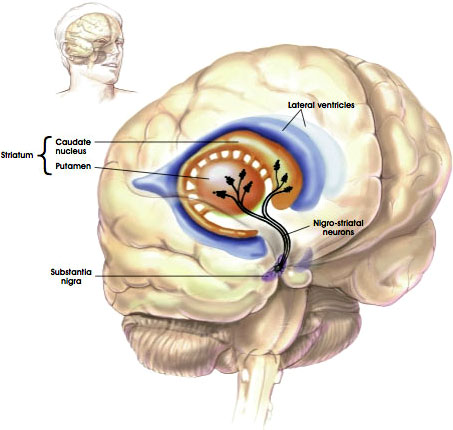
Source: NIH, n.d.
Lewy Bodies and Alpha-Synuclein
Lewy bodies are abnormal aggregates and inclusions of protein that develop inside nerve cells in people with Parkinson’s disease. The aggregations usually consist of insoluble fibrillary aggregates containing misfolded proteins. A large number of molecules have been identified in Lewy bodies but a protein called alpha-synuclein is the main component.
Lewy Bodies (Alpha-Synuclein Inclusions)
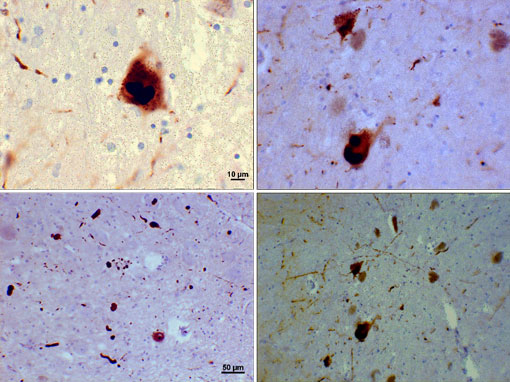
Photomicrograph of regions of substantia nigra in a Parkinson’s patient showing Lewy bodies and Lewy neurites in various magnifications. Top panels show a 60x magnification of the alpha-synuclein intraneuronal inclusions aggregated to form Lewy bodies. The bottom panels are 20× magnification images that show strand-like Lewy neurites and rounded Lewy bodies of various sizes. Images courtesy of Suraj Rajan.
Lewy pathology encompasses many regions of the brain and some reports have suggested that the substantia nigra is not the first place where Lewy bodies form in Parkinson’s disease. Inclusions and aggregates likely symbolize the end stage of a cascade of complicated events. An earlier stage may be more directly tied up to the pathogenesis of the disorder than the inclusions themselves, which may or may not represent diagnostic hallmarks.
Lewy bodies are also seen in “dementia with Lewy bodies,” suggesting that these conditions are related to one another by shared pathology and possibly by shared etiology. Neither cell loss nor the formation of Lewy bodies is absolutely specific for PD but both are required for a diagnosis of PD under current definitions (Cookson, 2009).
Neurodegenerative disorders such as Alzheimer’s disease, frontal-temporal degeneration, prion disease, Huntington’s chorea, and motoneuron diseases are increasingly being realized to have common cellular and molecular mechanisms, including protein aggregation and inclusion body formation in certain areas of the nervous system (Jellinger, 2011).
Inflammation and Immune Response
The trigger of dopaminergic degeneration seems to be multifactorial—affected by both endogenous and environmental elements. Inflammation and immune responses are increasingly being considered as important mediators of dopaminergic degeneration. Large population studies have suggested that individuals taking nonsteroidal anti-inflammatory drugs (NSAIDs) have less risk of developing idiopathic PD, which suggests that anti-inflammatory drugs may be a promising disease-modifying treatment for parkinsonian patients (Barcia, 2013).
New trial phases have involved anti-inflammatory treatments—specifically looking for an objective biomarker in treatments aimed at reducing inflammatory changes in patients with PD. Researchers are using neuroimaging tools to develop a relevant biomarker with the intention of testing this in large clinical imaging trials. The outcome of these trials will provide data to test and monitor the progression of anti-inflammatory treatments for PD and will help to identify the timely therapeutic window to stop, or at least slow, inflammatory-mediated dopaminergic degeneration (Barcia, 2013).
Parkinsonism
Parkinsonism, also known as “atypical Parkinson’s,” “secondary Parkinson’s,” or “Parkinson’s syndrome,” is a neurologic syndrome in which a patient exhibits some of the symptoms associated with Parkinson’s disease—tremor, rigidity, bradykinesia, and postural instability. But parkinsonism is not Parkinson’s disease. Parkinsonism is not thought to be caused by Parkinson’s disease and patients typically respond poorly to pharmacologic intervention. Parkinsonism often has an identifiable cause, such as exposure to toxins, methamphetamine, trauma, multiple strokes, other nervous system disorders, or illness. Generally, Lewy bodies are not seen in parkinsonism.
The term parkinsonism is also associated with disorders such as progressive supranuclear palsy, multiple system atrophy, Lewy body dementia, corticobasal degeneration, vascular parkinsonism, drug-induced parkinsonism, and parkinsonism secondary to infection and other causes (Hohler et al., 2012). A form of reversible parkinsonism can occur from the use of certain neuroleptic drugs, particularly reserpine, antipsychotics (haloperidol), and metoclopramide. Exposure to certain toxins, severe carbon monoxide poisoning, and mercury poisoning can also lead to parkinsonism.
The appearance in the early 1980s of parkinsonism symptoms in a group of drug addicts who had consumed a contaminated batch of a synthetic opiate led to the discovery of the chemical MPTP as an agent that causes parkinsonism syndrome in nonhuman primates as well as in humans. MPTP can be produced when making a form of heroin (MPTP is converted to a neurotoxin that selectively destroys dopamine cells in the substantia nigra). These cases are rare and have mostly affected long-term drug users.
Methamphetamine abuse has also been linked to parkinsonism. In experimental animals, exposure to methamphetamine damages dopaminergic fibers in the striatum* as well as the cell bodies in the substantia nigra, echoing the degeneration observed in human patients with PD. Selective damage to dopaminergic terminals in the striatum has also been observed in human methamphetamine users, although there is no evidence so far that methamphetamine abuse damages dopaminergic cell bodies in the substantia nigra (Granado et al., 2013).
*The largest nucleus of the basal ganglia, the striatum consists of the caudate nucleus and the putamen.
It has been hypothesized that methamphetamine use may predispose users to future development of PD. This hypothesis has been supported by recent epidemiologic work indicating that methamphetamine users have an increased risk of developing PD. This is consistent with the persistent neurotoxic effects of methamphetamine in experimental animals (Granado et al., 2013).
Patients with parkinsonism are often difficult to manage as outpatients. The complexity of their symptoms, the added cognitive and autonomic deficits, the poor response to most PD medications, and the relatively rapid decline in status contribute to the challenges in managing these patients, particularly as the disease progresses (Hohler et al., 2012).
