Because diagnosis is based on medical history, neurologic examination, and observation over time, a correct diagnosis is critical for effective management of the disease. Since many other diseases have similar features (especially when symptoms are mild), a timely and precise diagnosis is important so that patients can receive the proper and early treatment.
Brain scans and laboratory tests can be used to rule out other diseases but commuted tomography (CT) and magnetic resonance imaging (MRI) brain scans of people with PD usually appear normal. Cellular changes that occur on a microscopic, chemical level cannot be reliably detected by scans or blood tests.
Although there is progress on tests that can identify the presence of PD in vivo, Parkinson’s can currently only be definitively confirmed through its pathologic hallmark of Lewy bodies and Lewy neurites upon postmortem analysis (Haas et al., 2012). In the absence of confirming tests, the patient’s response to levodopa is often used to confirm the presence of PD.
There is a consensus among clinicians and researchers that new medical treatments for Parkinson’s disease should move from treating symptoms to modifying the disease pathology. The ultimate goal is to find neuroprotective treatments that stop or even prevent neurologic degeneration.
Symptomatic Treatment
Symptomatic Parkinson’s disease therapies are designed to alleviate motor and nonmotor symptoms, delay the progression of the disease, and manage the side effects of treatment. The challenge faced by clinicians is to find best treatments for each patient, re-evaluating as symptoms change. Among the many symptoms that occur in PD, cognitive changes, fatigue, anxiety and depression, sleep disturbances, and bladder and bowel dysfunction are usually treated successfully with a variety of drugs.
Early PD symptoms can be vague: increased clumsiness with the hands, mild gait irregularities, and intermittent tremor that is most obvious when the hand is resting or suspended when walking. Tremor, when present, is regular and rhythmic. A number of nonmotor symptoms such as loss of smell, sleep disturbances, sensory changes, and pain can occur well before motor symptoms are evident.
Dopamine Replacement
The pharmacologic mainstay for the treatment of Parkinson’s disease is the replacement of dopamine with levodopa, a precursor of dopamine. Dopamine replacement poses many challenges because only about 10% of a levodopa dose actually crosses the blood–brain barrier and enters the brain. The remaining levodopa is susceptible to conversion to dopamine in the periphery, leading to side effects such as nausea, dyskinesias, and joint stiffness. To address this, inhibitors that reduce the breakdown of dopamine in the peripheral nervous system—called peripheral dopa decarboxylase inhibitors (carbidopa and benserazide) are given in combination with levodopa to reduce peripheral conversion that would otherwise devour most of the dose given. The addition of dopa decarbozylase inhibitors also maximizes bioavailabilty of dopamine in the brain, decreases side effects, and allows a lower dose of levodopa to be used.
Once in the brain, as dopamine travels from one cell to another, it can be broken down and rendered inactive by two enzymes, MAO (monoamine oxidase) and COMT (catechol-O-methyl transferase). One therapeutic strategy introduces a MAO inhibitor into the synapse, which interrupts the action of the MAO enzyme and prevents the breakdown of dopamine in the synapse. This allows more dopamine to remain in the synapse and increases the likelihood that it will bind to the postsynaptic membrane.
Although levodopa helps in at least three-quarters of parkinsonian cases, not all symptoms respond equally to the drug. Bradykinesia and rigidity respond best, while tremor may be only marginally reduced. Problems with balance and other symptoms may not be alleviated at all. Controlled release versions of levodopa in the form of intravenous and intestinal gel infusions spread out the medication and are showing promise.
Initial drug treatment may start with MAO-B inhibitors and dopamine agonists. Levodopa plus a dopa decarboxylase inhibitor (such as carbidopa) are used sparingly at first to delay as long as possible the side effects resulting from cumulative exposure of systemic dopaminergic function.
As the disease progresses and dopaminergic neurons continue to be lost in the substantia nigra, L-dopa eventually becomes ineffective for treating the motor symptoms and may concurrently cause dyskinesias. As medication becomes less effective, “off” periods may occur when the levodopa dose has worn off and movement is again difficult until a new dose is given. Medications to treat nonmovement-related symptoms of PD, such as sleep disturbances and emotional problems, are also considered as needed (Goodman & Gilman, 2011).
After prolonged therapy with levodopa, a person with PD may alternate between phases with good response to medication and few symptoms (the “on” state) and phases with no response to medication and significant motor symptoms (the “off” state). Levodopa doses are therefore kept as low as possible, after using alternatives such as dopamine agonists and MAO-B inhibitors. Most people with PD will eventually require levodopa and hence later develop motor side effects such as involuntary movements (dyskinesia), painful leg cramps (dystonia), and a shortened response to each dose (motor fluctuations).
Transdermal Patches and Intestinal Gels
Transdermal dopaminergic patches is a recently developed therapy that has important advantages over pills and injectable medications. A patch formulation provides a more constant drug delivery, offers better compliance, avoids drug–food interactions, and offers the possibility of a once-a-day alternative. Additionally, pills may lose some clinical effectiveness when they are processed in the liver. The idea of a patch for a disease such as PD, where there are multiple drugs and multiple doses, is therefore very attractive to patients and to caregivers (Okun, 2012).
Duodopa is a new therapy recently out of clinical trials in the United States. Duodopa was approved for use in Europe in 2004. It may provide significant benefits by improving “on” time and reducing on-off fluctuations and dyskinesia. Duodopa is a pump-based therapy and requires the patient to wear a large external “box” in the belt region that is used to administer the intestinal gel preparation through a surgically placed intestinal tube.
Duodopa requires an attentive caregiver who must manage the device, the skin surrounding the tube, and medication refills. Early studies have revealed high rates of device-related problems with the intestinal tube (eg, clogging, kinking, moving out of the correct location). Despite these tube-related issues, Duodopa will likely be a great choice for many patients with on-off fluctuations, and will in most cases allow discontinuation of oral PD drugs (Okun, 2012).
Dopamine Agonists
Dopamine agonists are molecules that bind to the postsynaptic dopamine receptors and mimic the role of dopamine in the brain, causing a response similar to dopamine itself. Agonists were initially used to alleviate symptoms during the “off” state in patients with late PD when the benefits of levodopa doses were wearing off. Agonists are also used as an early alternative to levodopa so that later complications and dyskinesias are postponed for as long as possible.
|
Dopamine Agonists |
|
|---|---|
|
Generic name |
Brand name |
|
Bromocriptine |
Parlodel, Cycloset |
|
Pramipexole* |
Mirapex |
|
Ropinirole* |
Requip |
|
Piribedil |
Pronoran, Trivastal Retard, Trastal, Trivastan |
|
Cabergoline |
Dostinex, Cabaser |
|
Apomorphine |
Apokyn, Ixense, Spontane, Uprima |
|
Lisuride |
Dopergin, Proclacam, Revanil |
Dopamine agonists produce significant, though usually mild, side effects such as drowsiness, hallucinations, insomnia, nausea, and constipation. Agonists have also been related to impulse control disorders such as compulsive sexual activity, compulsive eating, and pathologic gambling and shopping. If side effects appear even at a minimal effective dose, another drug from this class can be tried as an alternative. These drugs are less effective than levodopa in the control of motor symptoms but are usually sufficient in the earliest stages of the disease.
Apomorphine may be used to reduce “off” periods and dyskinesia in late PD, though it requires injections or continuous subcutaneous infusions and may cause confusion and hallucinations. Apomorphine treatment obviously requires close attention from caregivers. Two other dopamine agonists are available as skin patches (lisuride and rotigotine) and have benefit in early stages and for the “off” state in advanced stages of PD.
MAO-B Inhibitors
Selegiline (Eldepryl, Deprenyl, or Selgene) and rasagiline (Azilect) are MAO-B inhibitors that increase the level of dopamine in basal ganglia synapses by blocking its metabolism. They inhibit the monoamine oxidase-B (MAO-B) enzyme responsible for breaking down dopamine. Like dopamine agonists, MAO-B inhibitors alone can improve motor symptoms and delay the need for levodopa early in the disease, but they are less effective than levodopa. In the advanced disease, they can be used to reduce fluctuations between “on” and “off” periods. None of these treatments slow the progression of the disease.
Other PD Treatment
Amantadine (Symmetrel) is a weak antagonist of NMDA-type glutamate receptors that increases dopamine release and blocks dopamine re-uptake in the synapse. It can be taken with levodopa to treat motor response fluctuations in advanced disease.
Anticholinergics that block the neurotransmitter acetylcholine in the central and peripheral nervous system may be useful to treat motor symptoms by essentially anesthetizing the muscle–nerve connections to reduce unwanted motor symptoms and rigidity.
Several drugs have been used to treat other symptoms common to PD patients, such as the use of clozapine (Clozaril, FazaClo) for psychosis, cholinesterase inhibitors for dementia, and modafinil for daytime sleepiness. Some studies have implied that regular users of non-steroidal anti-inflammatory drugs (NSAIDs, apart from acetaminophen and aspirin), have a lower risk of ever developing PD. Other medications are listed in the following table.
|
Other Drugs Used for Treating Parkinson’s Disease |
|
|---|---|
|
Drug |
Purpose |
|
Memantine (Namenda), rivastigmine (Exeleon), galantamine (Razadyne) |
Treatment of cognitive difficulties—these are NMDA receptor antagonists or acetylcholinesterase inhibitors. |
|
Antidepressants |
Treatment of mood disorders |
|
Gabapentin (Neurontin, Gralise, Fanatrex) |
Treatment of certain types of seizures or restless legs syndrome |
|
Duloxetine (Cymbalta) |
Treatment of depression, anxiety, peripheral neuropathy, fibromyalgia, or chronic pain related to muscles and bones. This is a selective serotonin and norepinephrine reuptake inhibitor (SSNRI). |
|
Fludrocortisone, midodrine, botox, sidenafil |
Treat of autonomic dysfunction
|
|
Armodafinil (Nuvigil), clonazepam (Klonopin), zolpidem (Ambien) |
Treatment of sleep disorders and daytime wakefulness |
Treatment of L-dopa Induced Dyskinesia
Levodopa remains the most effective agent to improve motor symptoms in PD but, as noted earlier, chronic use is associated with the emergence of motor fluctuations. This is manifested by a loss of clinical benefit before the next levodopa dose (wearing off), dyskinesias (abnormal involuntary movements), and nonmotor complications, such as behavioral and cognitive changes (Tambasco et al., 2012).
In most patients, L-dopa treatment begins with a “honeymoon” period during which motor symptoms are well controlled. However, after 5 years of treatment, approximately 40% of patients develop fluctuations in symptom control in response to the drug, as well as involuntary movements known as “L-dopa-induced dyskinesias” (LID). These complications affect as many as 89% of PD patients after 10 years of L-dopa treatment (Aviles-Olmos et al., 2012).
Dyskinesias usually improve when dopaminergic therapy is reduced but the reduction often cause PD symptoms to worsen. As the “off” state gets longer, bradykinesia usually increases, motor performance worsens, and daily activities are adversely affected.
Three risk factors are associated with increased occurrence of dyskinesias—younger age at disease onset, longer disease duration, and longer duration of dopaminergic treatment. The first two factors are interrelated and almost all patients with early-onset PD develop dyskinesias, whereas they are less frequent in patients with late-onset PD. Other risk factors associated with increased risk of dyskinesias are female gender and the occurrence of specific polymorphisms for dopamine receptors or dopamine transporters (Tambasco et al., 2012).
Peak Dose Dyskinesia
Dyskinesias more commonly appear as choreiform,* but in some cases they may resemble dystonia, myoclonus, or other movement disorders. Peak dose dyskinesias are the most common type of dyskinesia, which occur during peaks of levodopa-derived dopamine in the brain, when the patient is otherwise experiencing a beneficial response (the “on” state). Peak dose dyskinesias worsen with increases in dopaminergic dose and lessen when dopamine dose is reduced (Tambasco et al., 2012).
*Involuntary, irregular, dance-like movements that appear to move from one muscle to the next.
Diphasic Dyskinesia
In certain cases, dyskinesias appear with an alternating pattern (dyskinesia-improvement-dyskinesia). This is termed diphasic dyskinesia, and it tends to occur when levodopa-derived dopamine concentrations are increasing or decreasing. Diphasic dyskinesias are typically displayed with large-amplitude stereotypic, rhythmic, and repetitive movements, more often of the legs, that may be associated with parkinsonian features in other body regions. In extreme cases, patients treated with levodopa can cycle between “on” periods, which are complicated by disabling dyskinesias, and “off” periods, in which parkinsonism is uncontrolled and the patient is akinetic and frozen (Tambasco et al., 2012).
Motor complications occur in about 50% of patients with PD who have been in therapy with levodopa for more than 5 years, and in almost 100% of patients with young-onset disease. Achieving an acceptable clinical control once these motor fluctuations have appeared is usually a relatively simple matter, increasing the frequency of the levodopa doses or adding medications that reduce “off” time. However, when a patient develops peak dose dyskinesias too, it becomes difficult to smooth the clinical response. Although for many patients dyskinesias are not disabling, they create a barrier to adequate treatment of fluctuations and parkinsonian symptoms (Tambasco et al., 2012).
Alan: Living with Parkinson’s
My symptoms were worsening in 2010, and my quality of life was not what it had been. Dyskinesia, which is a common symptom of Parkinson’s, was the main problem. I could barely sit in a chair—I was constantly tipping over. On several occasions I even twisted right out of my office chair while at work.
It was when I saw two videos of myself that I started thinking seriously about having deep brain stimulation (DBS) surgery. One video showed me dancing with my daughter at her wedding; the other showed me speaking to the local Parkinson support group in early 2011. I was not aware how much my head was twisting until I saw those videos.
I was also experiencing an increasing number of Parkinson’s “spells.” The medical people call it “down time,” when your body does not respond to your medications. When I had a spell, I couldn’t walk but had to shuffle from place to place using one or two canes. I also had a fear of falling, which was happening a lot more often.
I decided to talk with my neurologist about having the DBS procedure. We had talked about the surgery as an option several times over the years. He had never recommended it because of the risks involved, but when I brought it up this time his attitude had changed.
“They are having a very high success rate these days,” he said.
Deep Brain Stimulation (DBS)
Deep brain stimulation (DBS) was approved by the FDA in 1997. It is recommended for people who have PD and suffer from motor fluctuations and tremor inadequately controlled by medication, or for those who are intolerant to medication, as long as they do not have severe neuropsychiatric problems (Okun, 2012). About 85,000 people worldwide have had DBS.
Deep Brain Stimulation Diagram
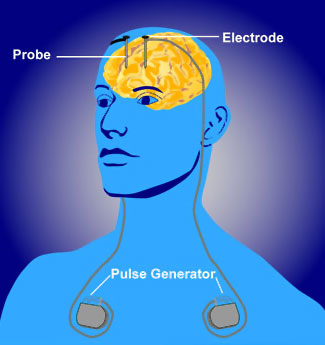
Source: NIMH, n.d.
Deep brain stimulation is a surgical intervention that utilizes an implantable pulse generator (neurostimulator) as a waveform generator and power source. The neurostimulator controls the flow of current to specific brain regions through an attachment to an implantable DBS lead. Each DBS lead has multiple contacts and therefore many possible parameter configurations. The optimization of possible settings, which may number into the thousands when considering the range of pulse widths, frequencies, amplitudes, and configuration of anodes and cathodes, can provide a critical determinant for therapeutic success or failure (Fakhar et al., 2013).
Deep brain stimulation is a two-stage procedure involving a stereotactic frame, with the patient under sedation yet awake, for a 30-minute, three-dimensional MRI to locate the coordinates of the deep brain target. After determining the target in the operating room, a path for the very fine metal electrodes is selected that will reach the target. The DBS electrode is placed, and electrical impulses are sent to see which placement gives the best reduction in tremors, while monitoring for other unwanted side effects in speech or numbness (Fakhar et al., 2013).
Once an effective place is found, the electrode is left in and clipped into place on the skull, and the exterior wound is closed. A second operation is performed under general anesthetic to place a small battery pouch containing the stimulator pulse generator under the collarbone. From there, a wire is passed under the skin up the neck to behind the ear, where it re-emerges and is attached to the stimulator wire in the brain. After observation for several weeks, the unit will be turned on and tested further. Depending on the targeted region of the brain, a neurologist will be involved with the delicate electrode placement, and one or both sides of the brain may be targeted, in similar but separate operations (Fakhar et al., 2013).
Determining Candidates for DBS
In deciding candidates for DBS, a good carbidopa/levodopa (Sinamet) profile is considered a key determinant for success. A person with a good Sinamet profile:
- Shows dramatic improvement in response to Sinamet
- Experiences a dramatic difference between “on” and “off” states
- Appears near normal in the “on” state
- Spends most of the day “off” (Larson, 2011)
Deep brain stimulation has shown good results with certain symptoms of PD while having little effect on other common symptoms. Dyskinesias and tremor are the symptoms most commonly helped. DBS can reduce on/off fluctuations (more “on” and less “off”) and can also address:
- Dyskinesias
- Tremor
- Stiffness
- Slowness of movement, including freezing episodes
- Shuffling gait (Larson, 2011)
Deep brain stimulation does not help:
- Swallowing problems
- Softness of speech
- Constipation
- Drooling
- Memory difficulties (Larson, 2011)
Alan: Living with Parkinson’s
To see if I was a good candidate for DBS, I had to undergo a series of tests. They began by videotaping me doing a series of movements such as walking, touching each of my fingers with my thumb, and standing up from a chair with my arms crossed in front of me. They made a video of me with no medication in my system and again after being medicated. The difference was major, but not surprising to me.
Another session was with a voice therapist. Again a video was taken of me doing various vocal exercises. Next was a swallowing test where I swallowed a variety of items, both liquid and solid.
The final session was a three-and-a-half hour neuropsychology exam. Each exercise started out simple but became more difficult.
I was mentally drained when it was over.
My surgeries were scheduled for August 2011. There were three in all. The first two were to open holes in my skull and put the wiring in place and the third was to install the device that creates the electrical impulse and connects everything together.
During the first two surgeries I was conscious. My skull was placed in a halo device to hold it steady. During the surgery the neurologist asked me to do certain movements at his command.
Someone asked me how it was to be conscious while the procedure was going on. I said that the hard part was hearing the drill as it bore through my skull.
Complications in Deep Brain Stimulation
Deep brain stimulation is associated with certain complications. Because an electrode penetrates the brain, there is a slight risk of puncturing small or medium-sized blood vessels. This occurs in 2% to 3% of cases, although permanent brain damage occurs in only 0.6% of cases, or 1 in 200. Infections occur in 4% to 5% of cases and may require removal of the hardware, although the brain electrodes are usually left in place. The most common site of infection is in the chest where the battery pack is located (Larson, 2011).
In less than 2% of cases, DBS has no effect and symptoms fail to improve, either due to malpositioned electrodes or because of an incorrect diagnosis. The success of DBS depends on a confident diagnosis and the choice of a good candidate. “Garden variety” PD responds well to DBS (Larson, 2011).
If other movement disorders that mimic PD are present, DBS is not effective. Red flags for the presence of something other than PD include more brain atrophy on MRI than is expected for a person’s age, evidence of severe vascular disease, or signs of other neurologic disease. A clinician should be suspicious of other neurologic disorders if these factors are present:
- Rapid onset of symptoms
- Rapid progression of symptoms
- Early onset of symptoms (early memory loss)
- Postural instability soon after diagnosis
- Autonomic failure soon after diagnosis
- Unusual findings on exam or on MRI (Larson, 2011)
Sinamet responsiveness is often used to determine the presence of PD, and a trial of this medication should clearly improve symptoms. Tests are performed before and after medication, and a 30% improvement after taking Sinamet is considered a good response and is usually correlated with a good response to DBS. Deep brain stimulation generally does not make symptoms better than a person’s best “on” state; rather, it tends to make “off” periods more like the “on” periods.
Degree of disability is important when considering DBS. Generally, it is not recommended in the early stages of PD when a patient is doing well on a consistent amount of medication that is controlling symptoms throughout the day. These patients are encouraged to wait, partly because the technology is improving rapidly. At the other end of the spectrum patients should not wait until symptoms have progressed so far that medications are ineffective (Larson, 2011).
Impaired Memory and Cognitive Function
Parkinson’s patients with impaired cognition generally do not do well with DBS, partly because the procedure is complicated and the patient must be able to reliably and clearly explain symptoms. Specific memory testing is now done on all patients to try to identify cognitive issues. If DBS is done in someone with memory problems, it is usually done only on one side of the brain and the patient is allowed to fully recover before the second implant is considered (Larson, 2011).
Age is also a consideration with DBS, although there is no cut-off age. Of concern is that with age the benefits associated with DBS decrease and the risk increases. Those over the age of 75 see only modest benefit and patients over the age of 80 are rarely offered DBS (Larson, 2011).
There are a number of other medical problems that increase risk of a poor outcome with DBS. Poorly controlled hypertension can make blood pressure difficult to control during surgery. Significant cardiac disease increases risk, especially in patients on blood thinners, which must be stopped a week before DBS surgery and remain stopped for a week after surgery. Other medical conditions such as diabetes or the use of steroid medications increase the risk of infection; however, this does not contra-indicate the DBS in many cases (Larson, 2011).
Long-Term Results Following DBS
Long-term results depend on which region of the brain receives DBS. Stimulation of certain areas of the brain primarily reduces limb tremor. Targeting other areas appears to reduce all of the major motor problems with PD, including those dyskinesias that arise after extended use of levodopa (Larson, 2011).
While the effects of DBS are not more effective than a dose of levodopa, it does seem to reduce the time spent in the “off” state and it allows a reduction in levodopa use so that side effects are pushed further into the future (Larson, 2011).
A study in Italy, which followed 14 patients for several years after DBS surgery, showed a 56% improvement after 1 year, a 45% improvement after 5 years, and a 42% improvement after 9+ years. The symptoms varied, however: tremors had the best sustained improvement, gait improved significantly after 1 year but declined over the next 8 years. Posture, balance, and ADLs (eg, rising from a chair) improved significantly after 1 year with no further improvement after 9 years (Larson, 2011).
Alan: Living with Parkinson’s
After the final surgery, I had to wait another week before the programming. That week seemed like an eternity.
But the day finally arrived. My neurologist turned on the device and started giving me the same commands he had given me in surgery. “Tap your foot, raise your leg, wave, turn the door knob, open and close your fist.”
Then came the real test.
“I want you to go out the door and walk down the hall.”
I did it without hesitation.
One of the nurses who had seen me wheeled in said, “It’s a miracle, he can walk again!”
Pallidotomy
Pallidotomy is a procedure in which a tiny electrical probe is placed in the globus pallidus (part of the basal ganglia), which is then heated to 80°C for 60 seconds, to ablate a small area of brain cells. Pallidotomy is an alternative to DBS for the treatment of levodopa-induced dyskinesia, and it can be an alternative to DBS for treating difficult cases of essential tremor.
Stem Cell Therapies
Stem cells are undifferentiated cells without mature, tissue-specific characteristics that are able to reproduce themselves by division into identical daughter cells. In response to proper stimuli, stem cells are able to produce more specific progenitor cells that can further differentiate into one or more functional cell types. Stem cells represent a very promising source of cell replacement therapy in a number of diseases, including PD, due to these key properties, namely, self-renewal and multipotentiality as well as the possibility to manipulate these cells in vitro (Jensen et al., 2011).
Dopaminergic neurons can be generated from stem cells of different sources. Embryonic stem cells (ESCs) have unlimited self-renewal capacity and are pluripotent, since they are able to generate cells of all three germ layers. Somatic (tissue-derived) stem cells can be isolated from developing tissues of the fetus or in the newborn, juvenile, or adult organism. Somatic stem cells have a more limited proliferation capacity than ESCs and are termed multipotent, typically being able to differentiate into the different cell types of one germ layer. Potential groups of stem cells for PD cell therapy include embryonic stem cells, neural stem cells, mesenchymal stem cells, and, more recently, induced pluripotent stem cells (Jensen et al., 2011).
The most important question regarding using stem cells as a therapy for PD remains whether it is possible to generate a large number of cells with the capacity to survive and function as dopaminergic neurons following transplantation; in addition, to ensure that these stem cell-derived grafts do not show adverse effects such as tumor formation or immune rejection (Jensen et al., 2011).
Types of Stem Cells
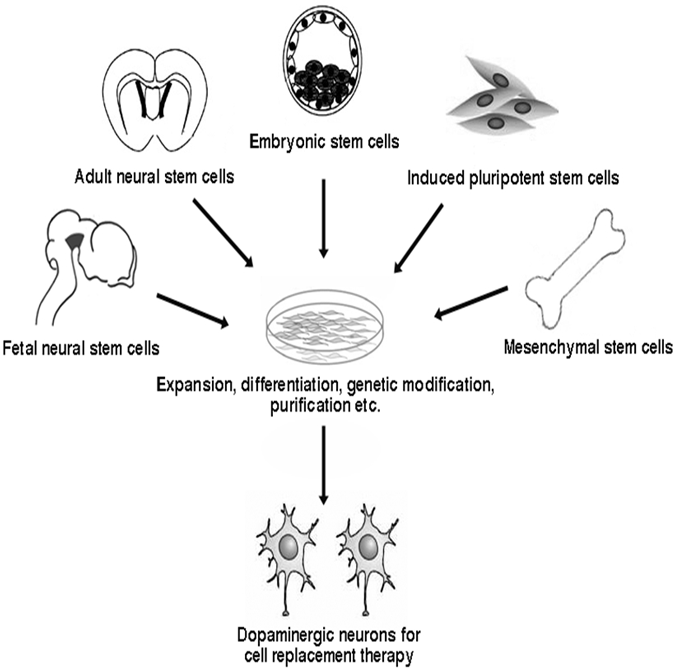
Source: Intechopen.com.
Since the 1980s fetal porcine carotid body cells or immature retinal tissues have been used in cell transplants, in which dissociated cells are injected into the substantia nigra in hope that they incorporate themselves into the brain and replace the dopamine-producing cells that have been lost. Though the results of dopamine-producing cell transplants were initially positive, further trials have not shown benefit beyond other types of current therapy. In some cases the new cells were secreting more dopamine than was necessary, leading to the dystonias common in advanced PD.
Stem cell transplants continue to be a research target, because stem cells are easy to grow and manipulate, and when transplanted into the brains of rodents and monkeys they have been able to survive and reduce abnormalities. Reprogramming of cells using pluripotent stem cells derived from the patient is being actively studied.
Several molecules have been proposed as potential treatments aimed at reducing the rate of degeneration in PD patients. None of them have been convincingly shown to reduce degeneration.
Online Resource
Ask the MD: Stem Cells and Parkinson’s Disease [4:17]
https://www.michaeljfox.org/news/ask-md-stem-cells-and-parkinsons-disease
Inhaled Levodopa
Clinical trials, partly funded by the Michael J. Fox Foundation, are underway on an inhaled formulation of levodopa. Called CVT-301, the therapy is designed to function as a sort of “rescue drug” to be taken in conjunction with the traditional pill form of levodopa/carbidopa (Sinemet). The idea is that patients taking CVT-301 could self-medicate by taking a puff from an inhaler should they feel an “off” period coming on. The medication is inhaled into the lungs and passes into the bloodstream much more quickly than oral medication.
In December 2018, the U.S. Food and Drug Administration (FDA) approved Inbrija™ (levodopa inhalation powder) for the intermittent treatment of “off” episodes in people with Parkinson’s disease who are already treated with carbidopa/levodopa. Inbrija, an inhaled version of levodopa, provides a new method of delivery for this medication.
