A vaccine is a substance (an antigen) made from a virus or bacterium that triggers the body’s immune system to develop antibodies. Substances are sometimes added to a vaccine to generate a stronger immune response so that less vaccine is needed for the body to recognize and fight the antigen. Influenza vaccines cause antibodies to develop about 2 weeks after vaccination.
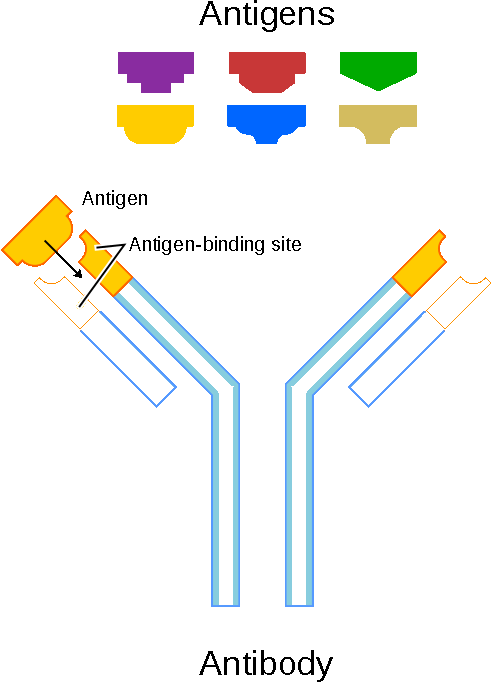
An illustration that shows how antigens induce the immune system response by interacting with an antibody that matches the molecular structure of an antigen. Antibodies bind with unwanted antigens and remove them from the body. Public domain.
In late February to early March—before the new flu season begins—an FDA advisory committee reviews data about which flu viruses have caused disease in the past year, how the viruses are changing, and disease trends, and recommends the three or four flu strains to include in the trivalent and quadrivalent influenza vaccines for the U.S. in the upcoming flu season (FDA, 2020, September 28).
There is often more than one type of influenza virus circulating each season, so influenza vaccines are formulated to target the most likely influenza viruses of the season: two influenza A types (H1N1 and H3N2) and one (trivalent vaccine formulation) or two (quadrivalent vaccine formulation) types of influenza B (FDA, 2020, September 28).
The most common way that flu vaccines are made is using an egg-based manufacturing process that has been used for more than 70 years. Egg-based vaccine manufacturing is used to make both inactivated (killed) vaccine used in the flu shot and live attenuated (weakened) vaccine used in the nasal spray flu vaccine (CDC, 2021b, August 31).
Types of Vaccines Available in the U.S.
Three types of influenza vaccine are available in the United States:
- Inactivated influenza vaccine (IIV)
- Live, attenuated influenza vaccine (LAIV)
- Recombinant influenza vaccine (RIV) (Hall, 2021).
Inactivated influenza vaccines (IIV) have been available since the 1940s and have traditionally been administered intramuscularly or intradermally. They are produced by killing the disease-causing microbe with chemicals, heat, or radiation. Inactivated vaccines are more stable and safer than live vaccines because the dead microbes cannot mutate back to their disease-causing state. However, most inactivated vaccines stimulate a weaker immune system response than do live vaccines (NAIAD, 2019, July 1).
Trivalent inactivated flu vaccine (TIV) containing 2 influenza A antigens and 2 influenza B antigen has, until recently, been the mainstay of seasonal flu vaccination programs. In 2012, the FDA approved the first quadrivalent flu vaccine containing an additional B antigen.
Quadrivalent inactivated flu vaccine (QIV) is designed to protect against four different flu viruses: two influenza A viruses and two influenza B viruses. All flu vaccines in the U.S. for the 2021-2022 season are quadrivalent vaccines. Different vaccines are approved for different age groups: there is a quadrivalent flu shot that can be given to children as young as 6 months old. Flucelvax Quadrivalent is now approved for people 2 years and older (CDC, 2021, August 27).
Live attenuated influenza vaccine (LAIV) was approved for use in the U.S. in 2003. These vaccines contain a version of the living microbe that has been weakened in the lab so it cannot cause disease. It does not contain thimerosal or any other preservative.
LAIV is provided in a single-dose sprayer unit; half of the dose is sprayed into each nostril. The weakened viruses are cold-adapted, which means they are designed to only multiply at the cooler temperatures found within the nose. The viruses cannot infect the lungs or other areas where warmer temperatures exist.
All nasal spray flu vaccines for the 2021-2022 season are quadrivalent. The nasal spray flu vaccine is approved for use in healthy non-pregnant people, 2 through 49 years old. People with certain medical conditions should not get the nasal spray flu vaccine (CDC, 2021 August 3).
Recombinant influenza vaccine (RIV) was first approved for use in 2013. The RIV manufacturing process uses recombinant DNA technology and does not require an egg-grown vaccine virus. The resulting vaccine contains recombinant hemagglutinin (Hall, 2021).
Recombinant technology can produce a vaccine in a shorter amount of time than either egg-grown or cell-grown technologies. The only influenza vaccine produced using recombinant technology is Flublok Quadrivalent. It has been licensed by the FDA for use in adults 18 years and older (CDC, 2021b, August 31).
Flublok Quadrivalent (RIV4) is available for the 2021–2022 influenza season for persons aged ≥18 years. Flublok RIV4 is manufactured without the use of influenza viruses so no shedding of vaccine virus will occur. This vaccine contains recombinant HA produced in an insect cell line using genetic sequences from cell-derived influenza viruses and is manufactured without the use of influenza viruses or eggs (Grohskopf et al., 2021).
For 2024–2025: For information on approved flu vaccines for the 2024–2025 flu season, please see Trivalent Influenza Vaccines for the 2024-2025 U.S. Influenza Season.
Influenza Vaccine Key Points | |
|---|---|
Inactivated influenza vaccine (IIV) |
|
Inactivated influenza vaccine (IIV) |
|
Live attenuated vaccine (LAIV) |
|
Recombinant influenza vaccine (RIV) |
|
Immunity Following Vaccination
Immunity following administration of inactivated influenza vaccine is less than 1 year, due to waning of vaccine-induced antibodies and antigenic drift of circulating influenza viruses. Influenza vaccine efficacy varies by the similarity of the vaccine strain to circulating strains and the age and health of the recipient.
CDC conducts studies each year to determine how well the influenza vaccine protects against flu illness. While vaccine effectiveness can vary, recent studies show that flu vaccination reduces the risk of flu illness by between 40% and 60% among the overall population during seasons when most circulating flu viruses are well-matched to the flu vaccine. In general, current flu vaccines tend to work better against influenza B and influenza A(H1N1) viruses and offer lower protection against influenza A(H3N2) viruses (CDC, 2021, October 25).
Pregnant Women and Neonates
Pregnant and postpartum women have been observed to be at higher risk for severe illness and complications from influenza, particularly during the second and third trimesters. Influenza vaccination during pregnancy is associated with reduced risk for respiratory illness and influenza among pregnant and postpartum women, as well as infants during the first several months of life (Grohskopf et al., 2021).
Although experience with the use of IIVs during pregnancy is substantial, data specifically reflecting administration of influenza vaccines during the first trimester are relatively limited. Most studies have not noted an association between influenza vaccination and adverse pregnancy outcomes, including spontaneous abortion (Grohskopf et al., 2021).
Substantially less experience exists with more recently licensed IIVs (e.g., quadrivalent and cell culture–based vaccines) during pregnancy as compared with previously available products. For RIV, data are limited (Grohskopf et al., 2021).
Older Adults
Because of the vulnerability of older adults to severe influenza illness, hospitalization, and death, and efficacy and effectiveness of influenza vaccines among older adults is an area of active research (Grohskopf et al., 2021).
Comparative studies of vaccine efficacy and effectiveness against laboratory-confirmed influenza outcomes among older adults have focused on HD-IIV3 (Fluzone High-Dose), RIV4 (Flublok Quadrivalent), and aIIV3 (Fluad). Each of these three vaccines has been studied in comparison to a standard dose, unadjuvanted IIV. Although HD-IIV3 has been the most extensively studied, evidence has accumulated for its superior efficacy and effectiveness compared with SD-IIV3 in this population. For the 2020–21 season, quadrivalent formulations of high-dose and adjuvanted influenza vaccines have been introduced; trivalent formulations of these vaccines will not be available for the 2021–22 season. (Grohskopf et al., 2021).
