After a stroke, many people experience motor and sensory impairment in the upper extremity. In contrast to lower extremity recovery, in which outcomes are more favorable, recovery of upper limb function after stroke is poor. In the Copenhagen Stroke Study for example, about a third (32%) of stroke patients had severe arm paresis at admission while more than a third (37%) had mild paresis. In 13%, the arm remained entirely non-functional despite comprehensive rehabilitation efforts (Foley et al., 2013b). These discouraging outcomes reflect the complex structure, range-of-motion, neurologic control, and function of the shoulder, arm, and hand.
The upper extremity is responsible for both reaching and grasping, and pathology affecting one often affects the other (Shumway-Cook & Woollocott, 2012). Motor control problems include:
- Delayed movement times
- Loss of ability to adapt to changing task demands
- Slowed reaction times (Shumway-Cook & Woollocott, 2012)
Upper extremity rehabilitation programs are typically designed and delivered by physical or occupational therapists, based on their assessment of movement impairment. The success of this approach depends on the skill and experience of the therapist and on the duration of treatment. However, there is no standard procedure for the assessment and treatment of the impairment in arm movement. This leads to the variability in the effectiveness of therapy and to the inability to compare interventions across practitioners and clinics (Olesh et al., 2014).
Additionally, current consensus is that physical therapy continues to be effective months and years after a neurologic damage such as stroke. However, with the current one-on-one hospital session approach, prolonged treatment is extremely expensive and usually does not last beyond the first month following a stroke (Olesh et al., 2014).
A variety of techniques and devices are used by rehabilitation therapists to treat patients with upper extremity dysfunction. Neurodevelopmental treatment (NDT) techniques, bilateral arm training, robot-assisted training, constraint-induced movement therapy, and neuromuscular electrical stimulation, to name a few, have all, to one degree or another, been shown to improve upper extremity function following a stroke. In general, research indicates that training should start as early as possible and be intensive, repetitive, and task-oriented (Brauer et al., 2013).
A review of the evidence for upper extremity interventions following stroke by Canadian researchers noted that the initial degree of motor impairment is the best predictor of motor recovery. Functional recovery goals are appropriate for those patients who are expected to achieve a greater amount of motor recovery in the arm and hand. Compensatory treatment goals should be pursued if there is an expected outcome of poor motor recovery. Attempts to regain function in the affected upper extremity should be limited to those individuals already showing signs of some recovery (Foley et al., 2013b).
For those with severe motor, sensory, and functional deficits in the involved limb after stroke, additional treatment for the upper limb will not result in any significant neurologic change. The evidence to date suggests that interventions may not lead to meaningful functional use of the affected limb, at least for those with severe deficits (Foley et al., 2013b).
Neurodevelopment Treatment (NDT)
There are a number of approaches that fall under the heading of neurodevelopmental techniques. These include the Bobath, Brunnstrom, and proprioceptive neuromuscular facilitation (PNF) approaches. Arguably, the Bobath approach is the most commonly used in the treatment of upper extremity impairment following a stroke (Foley et al., 2013b).
The motor control theory underlying neurodevelopmental treatment (NDT) emphasizes the concept that abnormal muscle patterns or muscle tone arise as a result of damage to the brain following a stroke. In order to inhibit abnormal tone, normal patterns must be practiced to facilitate functional and voluntary movements.
In several reviews of NDT vs. other treatment approaches, reviewers concluded that NDT was not superior to other types of interventions. A systematic review of specific neurologic treatment approaches also concluded that, compared to a Bobath (NDT) approach, no one particular program was favored over another with respect to improvement in functional outcomes (ADLs), muscle strength or tone, and dexterity, although motor relearning programs were associated with shorter lengths of hospital stays (Foley et al., 2013b). Despite this, theories based on neurodevelopmental theories continue to be used in the clinical setting.
Bilateral Upper Limb Training
Bilateral upper limb training involves the simultaneous use of both upper limbs with one limb moving actively and the other limb moving actively, passively, or with assistance (van Delden et al., 2012). This type of training is by no means a new form of stroke rehabilitation. Since days long past, therapists have used equipment (such as pulleys) to move the impaired upper limb simultaneously with the less impaired upper limb. Recently, there has been increased interest in bilateral upper limb training as new theories of neural plasticity have gained support (Foley et al., 2013b).
Theoretically, the use of the intact limb promotes functional recovery of the impaired limb through coupling between the upper limbs. Practicing bilateral movements may activate the intact hemisphere and facilitate the activation of the damaged hemisphere through neural networks linked via the corpus callosum (Foley et al., 2013b).
Coupling of upper extremity function is based on the following concepts:
- Neurally mediated dependencies between the right and left upper extremities
- Interhemispheric interactions along with bimanually triggered activation of similar neural networks in both hemispheres
- Training-related plasticity of the brain (Sleimen-Malkoun et al., 2011)
Coupling between the two upper limbs has been investigated extensively in healthy subjects using rhythmic, inter-limb movements. Healthy subjects have a tendency to organize their arm movements in a symmetrical manner (same movement with both arms) or in a coordinated, alternating manner (same movement with opposite arms). These tendencies reflect the coupling between the upper limbs (van Delden et al., 2012).
Bilateral upper limb training is often done with either manual or computerized devices, which can be simple in design or part of a sophisticated robotic device. There is some evidence that bilateral arm training may be most effective in the early stages following a stroke when brain reorganization is at its peak (Shumway-Cook & Woollacott, 2012). These devices provide good support, allowing for unlimited repetitions and a wide variety of movements. Some examples are described in the next section.
Recent systematic reviews have produced mixed results on the superiority or inferiority of bilateral upper limb training over other interventions used in post stroke rehabilitation. Two reviews found strong evidence in support of bilateral upper limb training after stroke while three other systematic reviews concluded that bilateral training is as effective as other treatments but not better (van Delden et al., 2012).
Manual Bilateral Training Devices
BATRAC
BATRAC Training Devices

Modified BATRAC apparatus. Reprinted with permission.
Bilateral arm training with rhythmic auditory cueing (BATRAC) was introduced in 2000, together with a custom-made bilateral arm trainer. The device consists of two independent T-bar handles mounted on nearly frictionless tracks that can move in the transverse plane perpendicular to the user. The handles have to be pushed forward and pulled back, either with both upper limbs simultaneously (in-phase) or alternately (anti-phase), at a frequency paced by a metronome providing auditory cues. If a patient is unable to hold the handle with the hand of the most impaired upper limb, the hand is strapped onto it. The original BATRAC protocol focuses expressly on shoulder and elbow function (van Delden et al., 2012).
Reha-Slide Duo
The Reha-Slide Bilateral Training Device

Reha-Slide Duo. Reprinted with permission (http://www.reha-stim.de/).
The Reha-Slide Duo consists of a board with two sledges running on parallel tracks. Two handles on the sledges can be moved forward and backward separately, similar to the Tailwind used for BATRAC. The board on which the tracks are placed can be inclined up to 20° for upward movements, and friction for forward and backward movements can be adjusted for both handles separately via adjustable rubber brakes (with the board horizontal) (van Delden et al., 2012).
The Rocker for APBT
The Rocker for APBT
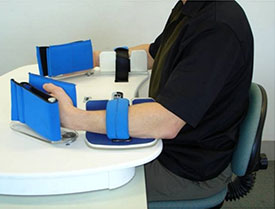
The Rocker for active-passive bimanual movement therapy. Reprinted with permission.
The device used for active-passive bimanual movement therapy (APBT) is a custom-built system of connected crankshafts located in the body of a unit that couples two manipulanda. It supports mirror symmetrical or near-symmetrical (a phase lag of 60°) coordination of wrist flexion and extension movements in the horizontal plane. With this system an actively moving less-impaired upper limb can passively move the most impaired upper limb in either a synchronous or (60° phase lag) asynchronous fashion (van Delden et al., 2012).
Robot-Mediated Bilateral Training Devices
Robot-mediated therapy for the treatment of upper limb impairment dates back to the 1990s. Since then a number of robotic devices have become commercially available to clinics and hospitals. Robotic devices can provide high-intensity, repetitive, task-specific, interactive training. Typically, such robots deliver forces to the paretic limb while practicing multi-joint gross movements of the arm. Most of the robotic devices applied in clinical trials or clinical practice offer the possibility of choosing among four modalities for training: active, active-assisted, passive, and resistive (Basteris et al., 2014).
There is evidence that robotic interventions improve upper limb motor scores and strength, but these improvements are often not transferred to performance of ADLs. These findings are shared among the most recent studies, including the largest randomized controlled trial related to robot therapy to date. A possible reason for a limited transfer of motor gains to ADLs is that the earlier studies on robot-mediated therapy have only focused on the proximal joints of the arm. Integration of distal with proximal arm training is now recognized as essential to enhance functional gains (Basteris et al., 2014).
A growing body of evidence has shown that large numbers of repetitions—carried out within intense and specific task-oriented training programs—are required to drive neuroplastic changes and to improve function. Robot-mediated training is highly repetitive in nature, which allows stroke patients—including those with severe impairment—to repeat movements hundreds of times. This is physically impossible using ordinary treatment methods. This feature is mostly due, in the most advanced systems, to the use of active robotic controllers. Performance-based algorithms enable the robot to adjust the mechanical assistance during the training session according to the patient’s motor performance. Most robotic devices use “assisted-as-needed” programs, the aim of which is to provide only as much assistance as the patient requires to complete the task (Duret et al., 2014).
Since the first clinical studies of the MIT-MANUS robot at the Massachusetts Institute of Technology, this innovative tool has continued to be studied clinically for the rehabilitation of the paretic upper limb, mainly after stroke. There are a multitude of studies of patients both in the acute and sub-acute phases of stroke recovery and in the chronic phase. The results are promising, showing that robotic therapy is safe and well tolerated and that it has a positive impact on improving motor impairments. These results led to the endorsement of the use of upper extremity robotics in the 2010 guidelines of the American Heart Association for Stroke Care (Duret et al., 2014).
Upper Limb Rehabilitation
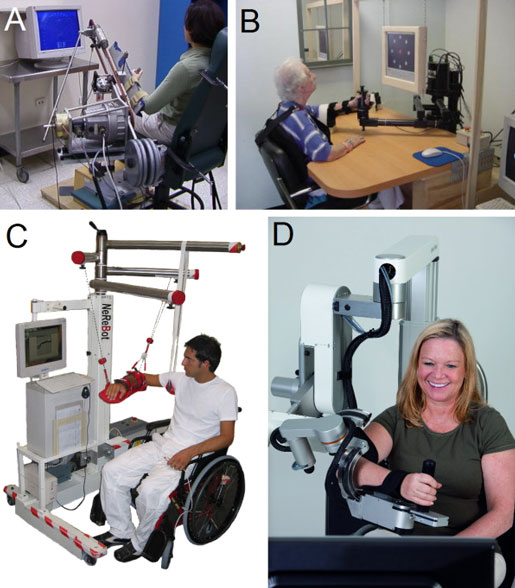
Examples of mechanical structures or robotic devices for upper limb rehabilitation. A: ARM Guide—a simple system using linear bearing to modify orientation; B: InMotion ARM—an end-effector-based commercial system; C: NeReBot—a cable-driven robot; D: ArmeoPower—an exoskeleton-based commercial system (courtesy of Hocoma AG).
There is strong evidence that sensorimotor training with robotic devices improves upper extremity functional outcomes and motor outcomes of the shoulder and elbow. There is also strong evidence that robotic devices do not improve motor outcomes of the wrist and hand (Foley et al., 2013b).
Strength Training
A small group of studies have evaluated treatments directed at increasing strength as opposed to function in the upper extremity. A much larger pool of studies has been published on strength training in the lower extremity. In a review of five studies that evaluated strength training and that assessed measures of strength, reviewers found strong evidence that strength training increases grip strength following stroke (Foley et al., 2013b).
Constraint-induced Movement Therapy
Constraint-induced movement therapy (CIMT) is a therapeutic strategy that forces the use of the affected arm by requiring a patient to perform functionally oriented activities while the non-paretic arm is physically restrained with a sling or glove. It is thought that the repetitive training of the paretic arm and constraint of the non-paretic upper arm might be important for promoting neural plasticity (Takeuchi & Izumi, 2013).
Constraint-induced Movement Therapy (CIMT)
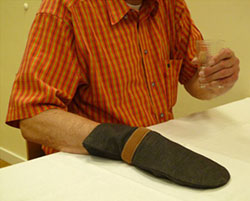
This figure illustrates one of the key therapeutic principles of CIMT: restriction of the less affected hand induces the patient to use the affected arm to drink a glass of water. Source: NIH, n.d.
Different categories of CIMT have been identified, depending on the duration of the immobilization and the intensity of task-specific practice: (a) original CIMT, (b) high-intensity modified CIMT, (c) low-intensity modified CIMT, and (d) immobilization of the non-paretic arm (“forced-use”) (Veerbeek et al., 2014).
Several studies reported neural plasticity after CIMT as evidenced by neuroimaging and neurophysiologic techniques. Previous studies using transcranial magnetic stimulation (TMS) found that the cortical representation size of the paretic hand was increased after therapy. Neuroimaging studies also demonstrated altered neural network activity after CIMT. Moreover, a structural magnetic resonance imaging (MRI) study reported that CIMT increased gray matter in the bilateral sensorimotor cortices compared with control therapy. Thus, there is evidence that CIMT induces both structural brain and physiologic changes in patients with stroke (Takeuchi & Izumi, 2013).
Although there is conflicting evidence of the benefit of CIMT in the acute stage of stroke, there is strong evidence of the benefit of modified CIMT in the acute/subacute stage of stroke. There is strong evidence of benefit of CIMT and modified CIMT in comparison to traditional therapies in the chronic stage of stroke. Benefits appear to be confined to stroke patients with some active wrist and hand movements, particularly those with sensory loss and neglect (Foley et al., 2013b).
Mirror Therapy
Mirror therapy is a technique that uses visual feedback about motor performance to improve rehabilitation outcomes. It has been adapted from its original use for the treatment of phantom limb pain as a method to “re-train the brain” as a means to enhance upper-limb function following stroke, and to reduce pain. In mirror therapy, patients place a mirror beside the unaffected limb, blocking their view of the affected limb, creating the illusion that both limbs are working normally (Foley et al., 2013b).
Mirror therapy is based on the theory that the recovery of skilled movement following a stroke requires accurate somatosensory function, in particular, light touch and proprioception. It appears that a relationship exists between the amount of sensory impairment and the degree of motor recovery. With somatosensory loss present in more than 60% of people with stroke, it is important that rehabilitation interventions target sensory as well as motor impairments because somatosensory function contributes to performance of ADLs following stroke (Kuys et al., 2012).
The effectiveness of mirror therapy was evaluated recently in a Cochrane review. The results from 14 RCT (567 subjects) were included. A modest benefit of treatment was reported in terms of motor function, but the treatment effect was difficult to isolate due to the variability of control conditions (Foley et al., 2013b).
Mirror therapy is a treatment for which there is a limited body of evidence in its application to stroke rehabilitation. There is conflicting evidence that mirror therapy improves motor function following stroke and moderate evidence that it does not reduce spasticity (Foley et al., 2013b).
Preventing Contracture
Spastic contracture following stroke is the expression of hypertonicity or increased active tension of the muscle. Contracture may also occur as a result of atrophic changes in the mechanical properties of muscles. Since surgery is the only treatment option once a contracture has developed, prevention is essential (Foley et al., 2013b).
Splinting
Splints have been widely used in clinical practice, with the aim of preventing contractures and reducing spasticity; however, splints have not been well studied. There is strong (Level 1a) evidence that hand splinting neither reduces the development of contracture nor reduces spasticity (Foley et al., 2013b).
Stretching
Stretching may help to prevent contracture formation and, although well accepted as a treatment strategy, it has not been well studied. There is moderate evidence that a nurse-led stretching program can help to increase range of motion in the upper extremity and reduce pain in the chronic stage of stroke (Foley et al., 2013b).
Electrical Stimulation
The application of electrical stimulation at a sensory level may help to enhance plasticity of the brain, which in turn may help with motor recovery. This is typically accomplished using a transcutaneous electrical nerve stimulator (TENS), which provides a current intensity beneath motor threshold, generating a “pins-and-needles sensation.” Similar to acupuncture, TENS is one method of achieving increased afferent (sensory) stimulation (Foley et al., 2013b).
By contrast, at a motor level, neuromuscular electrical stimulation can improve neuromuscular function in patients with stroke by providing an electrical current that is strong enough to cause a muscle contraction. This can be used to strengthen muscles, increase motor control, reduce spasticity, decrease pain, and increase range of motion. Neuromuscular electrical stimulation is generally categorized as either therapeutic electrical stimulation or functional electrical stimulation (FES) (Takeuchi & Izumi, 2013).
The defining feature of functional electrical stimulation is that it provokes a muscle contraction and produces a functionally useful movement during stimulation. Several upper extremity FES devices are available, and the use of these devices seems to have a positive effect on upper-limb motor function in both acute and chronic stages of stroke (Takeuchi & Izumi, 2013).
FES has also been combined with different walking training strategies and has been shown to result in improvements in hemiplegic gait in both acute and chronic stages of stroke (Takeuchi & Izumi, 2013). In the shoulder joint, FES is mainly used to stimulate those muscles that are responsible for maintaining the head of the humerus in the glenoid fossa—especially the supraspinatus and the posterior deltoid, which counteract the inferior displacement of the humerus and can therefore prevent or restore subluxation, reduce pain, and improve function (Vafadar et al., 2014). Another well-known application of FES is the peroneal nerve stimulator in patients with a drop foot Quandt & Hummel, 2014).
FES systems can be used to either substitute for or support movements and they are often applied in patients whose functional recovery has already plateaued. However, it has been shown that repeated muscle activation through FES might also lead to improvement of voluntary motor control beyond the time of stimulation, thus compounding the terms therapeutic and functional electrical stimulation and raising questions about exactly how FES influences motor recovery (Quandt & Hummel, 2014).
Numerous studies have found a positive impact of neuromuscular electrical stimulation for hand motor recovery after stroke, although strong evidence for its efficacy is still missing. A recent Cochrane review reported the superiority of electrical stimulation to no treatment; however, it could not identify an advantage over other treatment options such as conventional physical therapy (Quandt & Hummel, 2014).
Coupled Bilateral Training and Neuromuscular Stimulation
One effective rehabilitation protocol for those with post stroke upper extremity paresis involves coupled bilateral movements and neuromuscular stimulation on the more impaired arm. A study involving twelve volunteers with post stroke upper extremity weakness looked at the effects of neuromuscular stimulation on the impaired wrist and finger extensors, elbow extensors, and shoulder abductors. Subjects attempted to contract their impaired and weakened muscles while moving their other arm in the same motion (Kang et al., 2014).
Neuromuscular Stimulation
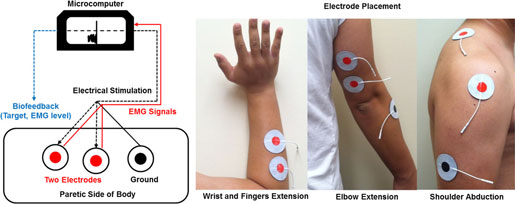
Experimental setup for providing electrical stimulation and recording EMG activation. Source: Kang et al., 2014.
The coupled bilateral movement training revealed more blocks moved, faster reaction time, greater force production, and higher peak limb velocity. Subjects attempted to contract their impaired and weakened muscles while moving their other arm in the same motion. Surface electrodes attached to the weakened muscles and microcomputer monitored activation levels. Once the muscle activity reached a target intensity level, the microcomputer automatically provided neuromuscular stimulation and movement was executed. The coupled bilateral movement training helped the impaired muscles perform basic movements (Kang et al., 2014).
When Upper Extremity Impairment Is Severe
Many of the techniques discussed in the previous section are effective for patients with mild to moderate weakness in an upper extremity. In more impaired patients, two issues limit the active involvement of the arm and hand in training:
- Reduced proximal arm function to transport the hand to a target, and
- Inability to actively open and close the hand once at the target (McCombe et al., 2014).
Studies focusing on more severe populations show promising results but are limited to proximal training approaches, which do not result in improvements in paretic hand function. To address these specific challenges, researchers at the University of Maryland developed a two-phase rehabilitation approach. Participants received either 6-weeks of bilateral proximal training followed sequentially by 6-weeks unilateral task-oriented training (COMBO) or 12-weeks of unilateral task-oriented training alone (SAEBO) (McCombe et al., 2014).
In Phase 1, participants received progressive bilateral arm training with rhythmic auditory cueing (BATRAC) for 6 weeks with a focus on improving proximal motor function and to potentially “prime” the central nervous system before transitioning in Phase 2 (McCombe et al., 2014).
Saeboflex Device for Hand Orthosis

The Saeboflex dynamic hand orthosis. Source Saebo.com. Used with permission.
In Phase 2, participants received unilateral task-oriented training using the Saeboflex dynamic hand orthosis to aid in active participation of the hand. Researchers compared this training approach to two 6-week sessions of time-matched unilateral task-oriented Saeboflex training with no proximal movement priming (McCombe Waller et al., 2014).
The results indicated that combining bilateral and unilateral task-oriented training improved arm and hand function more than unilateral task-oriented training alone in participants with moderate to severe chronic hemiparesis (McCombe et al., 2014).
Treatment of Edema
Hand edema with hemiparesis is associated with pain and stiffness, which can lead to a decrease in active motion and disuse. The etiology of the development of hand edema is unclear—it may be an isolated problem or occur as a symptom of shoulder-hand syndrome. The most widely accepted explanation is increased venous congestion related to prolonged dependency and loss of muscle pumping function in the paretic limb (Foley et al., 2013b).
Three different treatment approaches to aid in the reduction of hand edema following stroke have been studied, including passive motion exercises, neuromuscular stimulation, and intermittent pneumatic compression. There is moderate evidence that intermittent pneumatic compression does not reduce hand edema following stroke. There is limited evidence that both neuromuscular nerve stimulation and continuous passive motion help to reduce hand edema compared to limb elevation (Foley et al., 2013b).
