There’s no question that the Covid vaccines, which became widely available in spring 2021, have saved lives. The death rate for unvaccinated Americans peaked during the Omicron wave at around 26 per 100,000. For the vaccinated, that rate was 10 times lower, peaking around 2 per 100,000. For those with a booster dose, that rate was further halved.
J. Emory Parker, May 10, 2022 STAT
The ‘five pandemics’ driving 1 million U.S. Covid deaths
As we’ve all mentioned over and over again, there are approximately 70 million people in this country who have not been vaccinated. If you go back and look at the very, very many deaths that occurred back in 1918, the overwhelming majority of those deaths would have been avoided if back then, when my father was eight years old, they had a vaccine. But they didn’t. We do. And if we look at the people already who have died, the overwhelming majority of the people who’ve died from COVID-19 were people who were unvaccinated. Get vaccinated.
Anthony Fauci, September 28, 2021
Press Briefing by White House COVID-19 Response Team and Public Health Officials
An outbreak of a novel, sometimes deadly, severe respiratory disease emerged in China in early 2020. The virus was first seen in December of 2019 when a cluster of viral pneumonia cases was reported in Hubei Province. A novel coronavirus was identified as the causative agent of the pneumonia. The virus, temporarily named 2019-nCoV (now called SARS-CoV-2), caused a widespread epidemic throughout China.
With tens of thousands of coronavirus cases reported throughout the country, Chinese health officials took the unprecedented measure of quarantining nearly 60 million people. Despite the massive quarantines, the virus spread beyond China’s borders to other parts of the world.
Epidemiologists feared the outbreak had the potential to become a global health risk that might require a greater international response than Ebola, Zika, and H1N1 combined.
The United States reported its first confirmed case of person-to-person spread on January 30, 2020.
On January 31, 2020, the U.S President signed a “Proclamation on Suspension of Entry as Immigrants and Nonimmigrants of Persons who Pose a Risk of Transmitting 2019 Novel Coronavirus.”
On the same day, the World Health Organization (WHO) declared a public health emergency of international concern. WHO Director-General, Tedros Adhanom Ghebreyesus, explained:
The main reason for this declaration is not because of what is happening in China but because of what is happening in other countries. Our greatest concern is the potential for the virus to spread to countries with weaker health systems, which are ill-prepared to deal with it.
Despite these measures, by February 26, 2020 there were more confirmed COVID-19 cases outside of China than inside China. On March 11, 2020 the World Health Organization (WHO) declared a global pandemic.
On March 13, 2020, a national health emergency was declared in the United States. “The goal of the measures we have taken is to slow the introduction and the impact of the disease in the United States,” said Nancy Messonnier, director of the National Center for Immunization and Respiratory Diseases.
By late March 2020, the United States had surpassed China to become the country with the most confirmed cases of COVID-19. By mid-summer 2020, the United States was one of the hardest-hit countries; there were nearly 25% of global infections and 22% of global deaths from COVID-19.
In the Fall and Winter of 2020, as cases sored throughout the world, overwhelmed hospitals struggled to treat an increasing number of infected patients. Public health officials encouraged the use of rapid tests, masks, social distancing, and social isolation. Fad treatments were touted on social media and even by some doctors and politicians. Schools closed, sporting events were cancelled, and the term "essential worker" became part of our vocabulary. Researchers worked day and night to develop vaccines and therapeutics to treat the disease.
In late 2020, each week brought reports of records cases, deaths, and hospitalizations. In early December 2020, the first vaccines became available and vaccinations began for high-risk individuals and some essential workers. Concerns about COVID-19 variants began to appear in the news in the United Kingdom and South Africa along with a surge in deaths attributed to the variants.
In early 2021, as vaccines became more readily available, long lines formed outside health clinics, hospitals, and public health venues for the first dose of either the Pfizer or Moderna COVID-19 vaccines. Despite some vocal opposition to vaccination, some "hotspots" began to see a drop in COVID infections.
By early summer of 2021, it looked as though the Covid pandemic was in retreat in the United States. Due to the Delta variant, cases began to rise at an alarming rate, filling hospitals once again and closing recently reopened classrooms. From June to August 2021, the number of infections and deaths rapidly escalated, averaging over 150,000 cases per day. In late 2021, the Omicron variant emerged, once again overwhelming our healthcare system.
Despite the extraordinary speed of vaccine development against COVID-19 and continued mass vaccination efforts including guidelines recommending vaccine boosters, the continued emergence of new variant strains of SARS-CoV-2 threaten to overturn the significant progress made so far in halting the spread of SARS-CoV-2. As Dr. Anthony Fauci famously said in early September 2021, "People want to say we're done with COVID. But COVID's not done with us."
What Is a Coronavirus?
There are hundreds of coronaviruses, most of which circulate among animals, including pigs, camels, bats, and cats. Sometimes these viruses jump to humans (a spillover event) and can cause disease, as happened with Severe Acute Respiratory Syndrome (SARS), Middle East Respiratory Syndrome (MERS), and now COVID-19. Coronaviruses get their name from the characteristic crown-like spikes on the surface of the virus, which resembles a corona.
CoVID-19 Virus
SARS-CoV-2
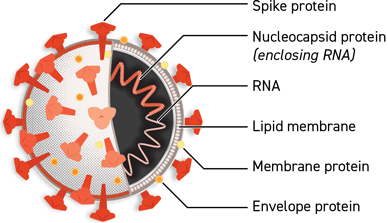
Left: Illustration of COVID-19 Coronavirus. Source: CDC, 2020. Right: Illustration of the SARS-CoV-2 structure. Source: Courtesy of Lisa Donohue, CoVPN, and COVID-19 Prevention Network. Used by permission.
There are four main sub-groupings of coronaviruses (alpha, beta, gamma, and delta). Only seven viruses in these sub-groups are known to cause human disease—four of which cause mild to moderate upper-respiratory illnesses such as the common cold.
However, three times in the twenty-first century, coronavirus outbreaks have emerged from animal reservoirs to cause severe disease and global transmission concerns: SARS (SARS-CoV-1), which emerged in late 2002 and disappeared by 2004; MERS, which was first identified in 2012 and consistently jumps from dromedary camels to people; and the novel coronavirus that emerged in December 2019.
While most coronaviruses only infect animals, MERS and SARS are notable for their ability to infect a variety of species, including humans. Recent research at the National Institute of Allergy and Infectious Diseases (NIAID) shows how the MERS virus can adapt to infect cells of a new species, which suggests that other coronaviruses may be able to do the same (NIAID, 2020, April 6). It appears that COVID-19 has done just that.
How Coronaviruses Adapt to Infect Other Species
To cause infection, a virus must first attach to a receptor molecule on a cell within the host species. This interaction is highly dependent on the shape of the receptors, which are determined by the host genes.
Colorizing a Virus

Visual artist Austin Athman shown making progress colorizing a SARS-CoV-2 image at NIAID’s Rocky Mountain Laboratories. Source: NIAID.
To evaluate how MERS evolved to infect host cells, scientists from NIAID tested 16 bat species and found that the virus was unable to enter cells with receptors from a common vampire bat, Desmodus rotundus. Scientists adapted the cells by growing virus on cells that had vampire bat receptors and observed the virus evolved to better infect the cells. After a few generations, the virus had completely adapted to the vampire bat’s receptors (NIAID, 2020 April 6).
By studying how the MERS virus changed over time in order to attach to the new host receptor, scientists found similarities with prior studies of the SARS virus. Thus, while these two viruses are different, they use essentially the same approach to enter the cells of new species (NIAID, 2020, April 6).
Human Coronaviruses
The new cluster of viral pneumonia cases originating in Wuhan, China, marks the third time in 20 years that a member of the large family of coronaviruses has jumped from animals to humans and sparked an outbreak.
Anthony Fauci
National Institute of Allergy and Infectious Diseases (NIAID)
There are seven coronaviruses that can infect people and cause illness. Four are fairly common and have circulated among humans since coronaviruses were first identified in the 1960s:
- 229E (alpha coronavirus)
- NL63 (alpha coronavirus)
- OC43 (beta coronavirus)
- HKU1 (beta coronavirus) (CDC, 2020 February 15)
These viruses have been circulating among humans for many years and usually cause the mild to moderate upper–respiratory tract illnesses like the common cold. Most people become infected with these viruses at some point in their lives and their illnesses are usually of short duration. Symptoms may include:
- Runny nose
- Headache
- Cough
- Sore throat
- Fever
- General feeling of being unwell (CDC, 2020 February 15)
Newer, more virulent human coronaviruses that infect animals and evolve to also infect humans are less common but more concerning:
- MERS-CoV (the beta coronavirus that causes Middle East Respiratory Syndrome)
- SARS-CoV (the beta coronavirus that causes severe acute respiratory syndrome)
- SARS-CoV-2 (the novel, beta coronavirus that causes coronavirus disease 2019, or COVID-19)
These human coronaviruses can also cause lower–respiratory tract illnesses, such as pneumonia or bronchitis; complications are more commonly seen in infants, older adults, and people with cardiopulmonary disease and/or weakened immune systems (CDC, 2020, February 15).
Coronavirus Symptoms
For confirmed COVID-19 infections, reported illnesses have ranged from people with little to no symptoms to people being severely ill and dying. Symptoms may appear 2 to 14 day after exposure. People with these symptoms or combination of symptoms may have COVID-19:
- Cough
- Shortness of breath or difficulty breathing
Or at least two of these symptoms:
- Fever
- Chills
- Repeated shaking with chills
- Muscle pain
- Headache
- Sore throat
- New loss of taste or smell
Symptoms differ with severity of disease. For example, fever, cough, and shortness of breath are more commonly reported among people who are hospitalized with COVID-19 than among those with milder disease. Atypical presentations occur often, and older adults and persons with medical comorbidities may have delayed presentation of fever and respiratory symptoms (CDC, 2020, June 30).
Scan of a Cell Infected with Coronavirus
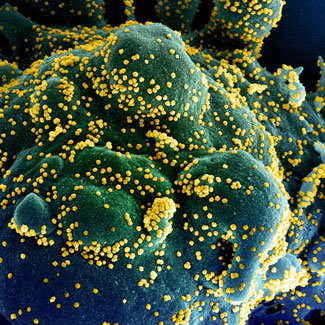
Colorized scanning electron micrograph of an apoptotic cell (blue/green) heavily infected with SARS-COV-2 virus particles (yellow), isolated from a patient sample. Image captured at the NIAID Integrated Research Facility (IRF) in Fort Detrick, Maryland. Source: NIAID.
Although the most common symptoms reported from COVID-19 are fever, dry cough, and fatigue, additional symptoms have been widely reported. Gastrointestinal symptoms can include nausea, vomiting, and diarrhea. Painful, itchy lesions on the hands and feet have been seen in younger people with less severe infection (sometimes referred to as COVID toes). Eye problems such as sensitivity to light, swollen eyelids, and watery eyes have also been reported.
In one study of 1,099 hospitalized patients, fever was present in only 44% at hospital admission but eventually developed in 89% during hospitalization. Fatigue, headache, and muscle aches (myalgia) are among the most commonly reported symptoms in people who are not hospitalized, and sore throat and nasal congestion or runny nose also may be prominent symptoms (CDC, 2020, June 30).
Many people with COVID-19 experience gastrointestinal symptoms such as nausea, vomiting, or diarrhea, sometimes prior to developing fever and lower respiratory tract signs and symptoms. Loss of smell (anosmia) or taste (ageusia) preceding the onset of respiratory symptoms has been commonly reported in COVID-19, especially among women and young or middle-aged patients who do not require hospitalization. While many of the symptoms of COVID-19 are common to other respiratory or viral illnesses, anosmia appears to be more specific to COVID-19 (CDC, 2020, June 30).
Several studies have reported that the signs and symptoms of COVID-19 in children are similar to adults, vary by age of the child, and are usually milder compared to adults (CDC, 2020, June 30).
As we move through the flu season in the Northern Hemisphere, it is helpful to understand the difference between influenza and COVID-19. Both are contagious respiratory illnesses, but they are caused by different viruses. COVID-19 is caused by infection with a coronavirus (called SARS-CoV-2) and flu is caused by infection with influenza viruses. Because some of the symptoms of flu and COVID-19 are similar, it may be hard to tell the difference between them based on symptoms alone, and testing may be needed to help confirm a diagnosis. Flu and COVID-19 share many characteristics, but there are some key differences between the two:
- Infection with COVID can cause a loss of taste or smell.
- COVID symptoms may take longer to develop than flu symptoms.
- People infected with COVID can be infectious for a longer period of time.
- COVID has produced more superspreading events than flu.
- COVID has caused blood clots in some individuals.
- Case of multi-inflammatory syndrome have occurred in children. (CDC, 2020, Jul 10)
Post-COVID Conditions
Some people who have been infected with the virus that causes COVID-19 can experience long-term effects from their infection, known as post-COVID conditions (PCC) or long COVID. People call post-COVID conditions by many names, including: long COVID, long-haul COVID, post-acute COVID-19, post-acute sequelae of SARS CoV-2 infection (PASC), long-term effects of COVID, and chronic COVID (CDC, 2022, May 5).
Fatigue is recognized as one of the most common presenting complaints in individuals infected with SARS-CoV-2. In an Irish study involving 128 participants, more than half reported persistent fatigue 10 weeks after initial COVID-19 symptoms. There was no association between COVID-19 severity and fatigue following infection. Additionally, there was no association between routine laboratory markers of inflammation and cell turnover or pro-inflammatory molecules and fatigue post COVID-19. Female gender and those with a pre-existing diagnosis of depression/anxiety were over-represented in those with fatigue (Townsend et al., 2020 November 9).
In one of the few reports to assess the long-term consequences of the severe acute respiratory syndrome (SARS) epidemic (caused by the novel coronavirus SARS-CoV), a subset of patients in Toronto experienced persistent fatigue, diffuse myalgia, weakness, and depression one year after their acute illness and could not return to work. In a similar follow-up study among 233 SARS survivors in Hong Kong, over 40% of respondents reported a chronic fatigue problem 40 months after infection. In those affected by the subsequent Middle-Eastern Respiratory Syndrome Coronavirus (MERS-CoV) outbreak, prolonged symptoms and fatigue were reported up to 18 months after acute infection (Townsend et al., 2020 November 9).
This research highlights the burden of fatigue, the impact on return to work, and the importance of following all patients diagnosed with COVID, not merely those who required hospitalization. There are enormous numbers of patients recovering from COVID infection worldwide. A lengthy post-infection fatigue burden will impair quality of life and have significant impact on individuals, employers, and healthcare systems. This highlights an emerging issue, which should be used to inform the management of convalescent patients and allow for timely interventions (Townsend et al., 2020 November 9).
Coronavirus Treatment and Management
For people at high risk of disease progression, the FDA has issued EUAs for a number of treatments for COVID-19. Monoclonal antibody treatments could help the immune system recognize and respond more effectively to the virus. Oral antiviral medications that target specific parts of the SARS-CoV-2 virus can help reduce its multiplication and spread through the patient’s body. Some of these treatments may not be effective against the Omicron variant. The NIH COVID-19 Treatment Guidelines provide recommendations about these treatments for COVID-19 and describe what is known about their effectiveness. If used, they should be administered as soon as possible after diagnosis (CDC, 2022, January 13).
Treatments can be used for different reasons, depending on the severity of the illness, in order to (CDC, 2022, January 13):
- Slow the virus. Antiviral medications reduce the ability of the virus to multiply and spread through the patient’s body.
- Reduce an overactive immune response. In patients with severe COVID-19, the body’s immune system may overreact to the threat of the virus, worsening the disease. This can cause damage to the body’s organs and tissues. Some treatments can help reduce this overactive immune response.
- Treat complications. COVID-19 can damage the heart, blood vessels, kidneys, brain, skin, eyes, and gastrointestinal organs. It also can cause other complications. Depending on the complications, additional treatments might be used for severely ill hospitalized patients, such as blood thinners to prevent or treat blood clots.
The incubation period for COVID-19 is thought to extend to 14 days, with a median time of 4-5 days from exposure to symptoms onset. One study reported that 97.5% of persons with COVID-19 who develop symptoms will do so within 11.5 days of SARS-CoV-2 infection. A large cohort of >44,000 persons with COVID-19 from China showed that illness severity can range from mild to critical:
- Mild to moderate (mild symptoms up to mild pneumonia): 81%
- Severe (dyspnea, hypoxia, or >50% lung involvement on imaging): 14%
- Critical (respiratory failure, shock, or multiorgan system dysfunction): 5% (CDC, 2020, June 30)
Mild Disease
Patients with a mild clinical presentation (absence of viral pneumonia and hypoxia) may not initially require hospitalization, and many patients will be able to manage their illness at home. The decision to monitor a patient in the inpatient or outpatient setting should be made on a case-by-case basis. This depends on the clinical presentation, requirement for supportive care, potential risk factors for severe disease, and the ability of the patient to self-isolate at home. Patients with risk factors for severe illness should be monitored closely given the possible risk of progression to severe illness, especially in the second week after symptom onset (CDC, 2020, June 30).
Severe Disease
Some patients with COVID-19 will have severe disease requiring hospitalization for management. Inpatient management revolves around the supportive management of the most common complications of severe COVID-19: pneumonia, hypoxemic respiratory failure/ARDS, sepsis and septic shock, cardiomyopathy and arrhythmia, acute kidney injury, and complications from prolonged hospitalization, including secondary bacterial infections, thromboembolism, gastrointestinal bleeding, and critical illness polyneuropathy/myopathy (CDC, 2020, June 30).
Some patients with COVID-19 may develop signs of a hypercoagulable state and be at increased risk for venous and arterial thrombosis of large and small vessels. Laboratory abnormalities commonly observed among hospitalized patients with COVID-19-associated coagulopathy include:
- Mild thrombocytopenia
- Increased D-dimer levels
- Increased fibrin degradation products
- Prolonged prothrombin time
Elevated D-dimer* levels have been strongly associated with greater risk of death (CDC, 2020, June 30).
*D-dimer: D-dimer is a protein fragment that is made when a blood clot has formed and is in the process of breaking down. A positive test indicates that there may be significant blood clot formation and breakdown in the body.
There are several reports of hospitalized patients with thrombotic complications, most frequently deep venous thrombosis and pulmonary embolism. Other reported manifestations include:
- Microvascular thrombosis of the toes
- Clotting of catheters
- Myocardial injury with ST-segment elevation
- Large vessel strokes (CDC, 2020, June 30)
The pathogenesis for COVID-19-associated hypercoagulability remains unknown. However, hypoxia and systemic inflammation secondary to COVID-19 may lead to high levels of inflammatory cytokines and activation of the coagulation pathway. There are limited data available to inform clinical management around prophylaxis or treatment of venous thromboembolism in COVID-19 patients (CDC, 2020, June 30).
Illness among pediatric patients with COVID-19 is typically milder than among adults. Most children present with symptoms of upper respiratory infection. However, severe outcomes have been reported in children, including deaths. Data suggest that infants (<12 months of age) may be at higher risk for severe illness from COVID-19 compared with older children. CDC and partners are also investigating reports of multisystem inflammatory syndrome in children (MIS-C) associated with COVID-19 (CDC, 2020, June 30). General treatment considerations include:
- Management of comorbid conditions.
- Prevention of pulmonary coinfections and superinfections, such as hospital-acquired pneumonia (HAP) and ventilator-associated pneumonia (VAP).
- Assessing treatable causes of shock such as bacterial sepsis, hypovolemic shock, cardiac dysfunction, or comorbid atherosclerotic disease and stress-related adrenal insufficiency.
- Management of COVID-19–induced cardiac dysfunction, including myocarditis.
- Evaluation and management of thrombotic events.
- Evaluating and managing renal and hepatic dysfunction.
- Understanding special considerations in children such as multisystem inflammatory syndrome in children.
- Evaluating drug-drug interactions.
- Monitoring risk for nosocomial infections and other complications of critical illness care, such as VAP, HAP, catheter-related bloodstream infections, and venous thromboembolism. (CDC, 2020, July 30)
