Before the current COVID-19 pandemic, three historically important epidemics had occurred since 2000: severe acute respiratory syndrome (SARS-CoV-1) in 2003, Middle East respiratory syndrome (MERS) in 2013, and Ebola virus disease in 2014. The first two were caused by coronaviruses and the third by ebolavirus. All three were eventually contained using now-familiar public health measures.
Why Learn About SARS, MERS, and Ebola?
In the West, we often shrug off what we consider to be exotic epidemics in regions of the world little-known to most of us. But in 2014, a great deal of uproar ensued when two Ebola-infected aid workers from a clinic in Liberia came down with Ebola and were transferred to Emory University Hospital in Atlanta for treatment. Many people raised fears of the virus spreading throughout this country. Both aid workers recovered.
Shortly thereafter, a man returned home to Texas from Sierra Leone by commercial airline. He became sick with Ebola about 5 days after he arrived home. Epidemiologists immediately began contact tracing, introduced earlier as a surveillance technique in which health officials find everyone who has been in direct contact with the Ebola patient. Contacts are watched for 21 days from the last day they were in touch with the Ebola patient. If a contact develops symptoms of Ebola, the new patient is isolated, tested, and provided with supportive care while all of his or her contacts are found and watched for 21 days.
It is striking to note that no matter where (or when) an outbreak occurs, we in the United States face many of the same difficulties other countries have faced. Distrust of science, lack of political leadership, limited resources, environmental destruction, and refusal to follow public health guidelines have greatly contributed to the spread of pandemic viruses.
SARS-CoV-1 (2003–2004)
Severe acute respiratory syndrome (SARS) is a viral respiratory illness caused by a highly pathogenic beta coronavirus, called SARS-associated coronavirus (SARS-CoV). Now referred to as SARS-CoV-1, it was an atypical pneumonia first detected in eastern China during the winter of 2002–2003. Symptoms included fever, chills, and body aches that usually progressed to pneumonia. Classic public health measures of isolation and containment brought the outbreak to an end. No human cases of SARS-CoV-1 have been reported anywhere in the world since 2004.
The SARS-CoV-1 outbreak brought back memories of the influenza pandemic of 1918–1919 in which as many as 50 million people died worldwide. The World Health Organization (WHO) called for immediate action against SARS and directed its Global Outbreak and Response Network in Geneva, Switzerland to coordinate efforts to monitor the spread of SARS throughout the world.
Bronchitis Viral Particles
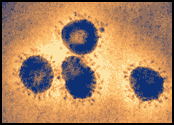
Infectious bronchitis virus particles, a coronavirus in the same family as SARS-CoV, as seen in a colorized electron microscopic image. Virions contain characteristic club-like projections emanating from the viral membrane. Source: F.A. Murphy and S. Whitfield, CDC.
The illness spread to more than two dozen countries in North America, South America, Europe, and Asia before the SARS global outbreak of 2003 was contained. There were more than 8,000 recorded cases, including 774 deaths, and the pandemic cost the global economy billions of dollars.
The 2003 SARS outbreak provided a modern example of how to contain a global epidemic using traditional or nonmedical public health measures. Interventions included finding and isolating case-patients; quarantining contacts; measures to “increase social distance,” such as canceling mass gatherings and closing schools; recommending that the public augment personal hygiene and wear masks; and limiting the spread of infection by domestic and international travelers by issuing travel advisories and screening travelers at borders (Bell, 2004).
Some measures were implemented pursuant to recommendations of the World Health Organization; others were implemented by governments on their own initiative. A novel technology, infrared scanning, was used extensively in some countries to try to identify persons with fever at international borders and in public places. After the outbreaks, WHO sought information to help assess the effectiveness of interventions in preventing the transmission of SARS both in the community and internationally (Bell, 2004).
Public campaigns to accelerate reporting and evaluating symptomatic patients appeared to decrease the interval between onset of symptoms and isolation of ill patients in several areas. Novel interventions included:
- Urging the entire population of affected areas to measure their temperature at least once daily
- Fever telephone hotlines
- Fever evaluation clinics with appropriate infection control measures
Thermal scanning in public places was implemented in several areas where community transmission was suspected. Data on the effectiveness of this practice are not available, but in Beijing thermal screening did not prove to be an efficient way to detect cases among intercity travelers (Bell, 2004).
Measures to increase social distance, such as canceling mass gatherings; closing schools, theaters, and public facilities; and requiring masks for all persons using public transport, working in restaurants, or entering hospitals, were implemented in areas where extensive unlinked community transmission of SARS coronavirus was suspected. Many persons in these areas also chose to wear masks outside their homes (Bell, 2004).
These measures were often applied simultaneously with other measures, including enhanced contact tracing, which makes their independent effectiveness difficult to assess. However, the simultaneous introduction of a variety of measures was temporally associated with dramatic declines in new SARS cases (Bell, 2004).
A case-control study in Beijing found that wearing a mask more frequently in public places may have been associated with increasing protection. Another case-control study in China–Hong Kong found that using a mask “frequently” in public places, washing hands >10 times per day, and “disinfecting living quarters thoroughly” appeared to be protective. The types of masks used were not specified (Bell, 2004).
In some areas, disinfectants were applied inside the homes and vehicles of persons with SARS, on ambulance tires, and over pedestrian walking zones. Little information exists on the effectiveness of disinfectant use in reducing community or hospital transmission. In Hong Kong, disinfecting living quarters thoroughly (not otherwise defined and reported retrospectively by telephone) appeared to be protective (Bell, 2004).
Travel advisories, along with advice to postpone nonessential travel, were issued by WHO and various governments. Air travel to areas affected by the advisories decreased dramatically during the epidemic, although the impact of advisories compared with other sources of information to travelers, such as news media reports of SARS cases, is difficult to assess (Bell, 2004).
SARS-CoV-1 was contained in human populations in 2003 largely by aggressive use of traditional public health interventions: case finding and isolation, quarantine of close contacts, and enhanced infection control measures in settings where care was provided to persons with SARS, especially in healthcare facilities and homes. These measures also contained a smaller SARS outbreak in 2004 that originated from a laboratory-acquired infection. Measures to decrease the interval between onset of symptoms and isolation were effective in containing community transmission. It should be noted that presymptomatic transmission was not observed and infectivity was low at the onset of illness (Bell, 2004).
MERS-CoV (2012)
Middle East Respiratory Syndrome (MERS) is a beta coronavirus believed to have jumped from animals to humans in 2012. The first case was believed to occur in Jordan in April 2012. A second, larger outbreak occurred in the Republic of Korea in 2015 (associated with a traveler from the Arabian Peninsula). So far, all cases of MERS have been linked through travel to, or residence in, countries in and near the Arabian Peninsula (CDC, 2019).
MERS-CoV
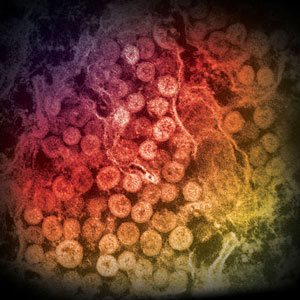
An electron micrograph of a thin section of MERS-CoV, showing the spherical particles within the cytoplasm of an infected cell. Source: Cynthia Goldsmith/Azaibi Tamin, CDC.
MERS caused severe respiratory illness with symptoms of fever, cough, and shortness of breath. The virus spread from ill people to others through close contact, such as caring for or living with an infected person. Patients ranged in age from younger than 1 to 99 years old.
About 3 or 4 out of every 10 people reported with MERS died. Most of the people who died had a pre-existing medical condition that weakened their immune system or an underlying medical condition that had not yet been diagnosed (CDC, 2019).
A wide clinical spectrum of MERS-CoV infection has been reported, ranging from asymptomatic infection to acute upper respiratory illness, and rapidly progressive pneumonitis, respiratory failure, septic shock, and multi-organ failure resulting in death. Most MERS-CoV cases have been reported in adults (median age approximately 50 years, male predominance), although children and adults of all ages have been infected. Most hospitalized MERS-CoV patients have had chronic co-morbidities. Among confirmed MERS-CoV cases reported to date, the case fatality proportion is approximately 35% (CDC, 2019).
Laboratory findings at admission may include leukopenia, lymphopenia, thrombocytopenia, and elevated lactate dehydrogenase levels. Co-infection with other respiratory viruses and a few cases of co-infection with community-acquired bacteria at admission have been reported; nosocomial bacterial and fungal infections have been reported in mechanically ventilated patients. MERS-CoV virus can be detected with higher viral load and longer duration in the lower, compared to the upper, respiratory tract and has been detected in feces, serum, and urine (CDC, 2019).
Duration of MERS-CoV shedding in the respiratory tract is typically longer in more severely ill patients than mildly ill patients, and evidence of virus has been detected in survivors for a month or more after onset. Limited data are available on the duration of extrapulmonary MERS-CoV shedding (CDC, 2019).
Unlike SARS, which was eliminated within several months of the initial outbreak, MERS continues to smolder due to sporadic transmission from camels—the virus’s intermediate host—to people, and limited chains of person-to-person transmission.
Ebola Virus Disease (2014)
In 2014 Ebola virus disease, also called Ebola hemorrhagic fever, emerged in West Africa, causing the largest outbreak of Ebola ever recorded. What began as a single case in the West African nation of Guinea quickly spread to neighboring Sierra Leone, Liberia, Senegal, and Nigeria with devastating impact. A lack of healthcare services, governments weakened by decades of civil war, and grossly inadequate infection prevention procedures and equipment hampered efforts to contain its spread. Distrust of the government, misconceptions about how Ebola spreads, and in some cases disbelief that Ebola even exists prevented public health efforts to contain the disease. The inability to isolate and trace those who had come in contact with the virus were chief concerns.
2014 Ebola Outbreak in West Africa—Distribution
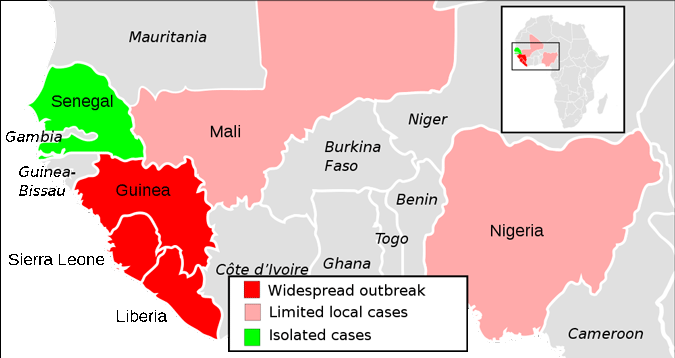
A map showing the extent of the Ebola virus epidemic in West Africa. Source: ZeLonewolf / CCO / Public domain.
Although undeniably deadly, previous outbreaks of Ebola were effectively contained using strict infection control, surveillance, and isolation procedures. There is evidence that sporadic, unrecognized or misdiagnosed, outbreaks with low levels of secondary transmission may occur relatively frequently (Kinsman, 2012).
Whether outbreaks are small or large, they have a profound psychological impact on the people living and working in the affected areas. “Alarm and near panic” were reported among health workers at Maridi Hospital in Sudan in 1976—an understandable reaction given that 61 of the hospital’s 154 nursing staff had fallen ill, of whom 33 then died (Kinsman, 2012).
During a 1995 epidemic in Kikwit, Zaire, health workers constituted 25% of the 315 Ebola cases, and fear of infection led many to quit their posts. Those who stayed at work subsequently reported feeling stigmatized because many people feared that they might act as carriers of the virus into the wider community. In some cases, neighbors threw stones, while others were chased from their houses (Kinsman, 2012).
The 2014 Ebola epidemic had a similar effect. When compared to previous Ebola outbreaks, however, the epidemic did not quickly abate. On September 30, 2014 the first laboratory-confirmed, travel-associated case of Ebola was reported in the United States. The traveler did not have symptoms on the flight back from West Africa and, fortunately, Ebola is contagious only if the person has active symptoms.
During the spring and summer of 2014, the World Health Organization, which one would expect to be at the center of efforts to control the spread of Ebola, was harshly criticized for its lack of response. In a September 4, 2014 New York Times article, reporter Sheri Fink noted the Ebola epidemic “has exposed gaping holes in the [Word Health Organization’s] ability to tackle outbreaks in an increasingly interconnected world, where diseases can quickly spread from remote villages to cities housing millions of people” (Fink, 2014).
With a lack of response by the World Health Organization, local hospitals and clinics struggled to contain new infections. The lack of a coordinated effort, the failure of WHO to quickly provide much-needed supplies and support, meant that healthcare workers were unable to protect themselves against infection. Many healthcare workers died as a result (Nossiter & Solomon, 2014).
One deputy nurse matron in Sierra Leone, Josephine Finda Sellu, who continued to work despite the dangers, lost 15 of her nurses to Ebola in rapid succession during June and July of 2014. Although the hospital has extensive experience dealing with Lassa fever, another type of hemorrhagic fever, and has modern infection prevention procedures in place, it was simply overwhelmed by as many as 80 new Ebola patients each day.
The most comprehensive early efforts to address the Ebola epidemic were undertaken by Doctors Without Borders. Joanne Liu, the organization’s director at the time, criticized WHO’s efforts and called on the United Nations to encourage countries with experience in biologic threats to set up mobile laboratories and field hospitals to treat Ebola patients. “It is your historic responsibility to act,” Dr. Liu said. “We cannot cut off the affected countries and hope this epidemic will simply burn out. To put out this fire, we must run into the burning building” (Sengupta, 2014).
Ebola Virus Particle
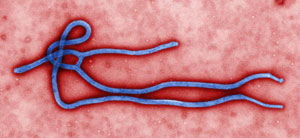
Digitally-colorized, transmission electron microscopic image which demonstrates the filamentous, branching structure of an Ebola virus particle. Source: CDC / Cynthia Goldsmith.
Although significantly less contagious than many viral diseases (particularly airborne disease such as measles, diphtheria, pertussis, and COVID-19), Ebola virus is nevertheless highly contagious. Once a person is exposed, symptoms may appear anywhere from 2 to 21 days after exposure—although 8 to 10 days is most common. Some who become sick with Ebola recover, while others do not. The reasons for this are not yet fully understood. However, it is known that patients who die usually have not developed a significant immune response to the virus at the time of death.
The clinical presentation of viral hemorrhagic fever is often nonspecific, with frank bleeding seen in a minority of cases—so cases may be mistaken for other more common diseases or, in the case of Guinea, Lassa fever, which is endemic in the area of the outbreak. Nor are laboratory diagnostics routinely available in West Africa for most viral hemorrhagic fevers. Ebola virus testing of human serum samples collected as far back as 1996 as part of surveillance for Lassa fever in the same region as the current outbreak could help reveal whether humans had exposure to Ebola virus prior to this outbreak (Bausch & Schwarz, 2014).
Fever, coughing, severe headache, muscle and joint pain, vomiting, diarrhea, loss of appetite, abdominal pain, shortness of breath, chest pain, and a maculopapular rash (a skin rash consisting of discoloration and raised spots) are common early symptoms of Ebola infection. These clinical features are strikingly similar to many other diseases endemic to Africa making early identification difficult. Nevertheless, early recognition is crucial for infection control and treatment.
Learning about Ebola is important on two counts: (1) it allows us to identify an infectious disease that is unfamiliar, which means treatment can begin early in the course of the disease; and (2) it also helps us prevent the spread of erroneous information.
Surveillance During the Ebola Pandemic
A common characteristic of large Ebola and Marburg viral disease outbreaks is the breakdown (or absolute lack of) public health surveillance, resulting in long periods of time before public health officials can identify the outbreak. With aggressive surveillance, early chains of transmission can be identified and outbreak response efforts rapidly applied (MacNeil & Rollin, 2012). For example, during the reemergence of Ebola viral disease in Luwero district, Uganda in 2011, viral hemorrhagic fever was immediately suspected in the index (and only case) by clinicians at the hospital. Luwero is in a rural area less than 2 hours by vehicle from the capital of Uganda (Kampala).
A confirmatory laboratory diagnosis was acquired in less than a week, and outbreak response activities started within 24 hours. While contacts of this Ebola case fortunately did not develop disease, the ability to identify and followup all contacts would have resulted in prevention of further spread of the virus, should secondary cases have developed (MacNeil & Rollin, 2012).
Laboratory diagnostics are a crucial component of public health surveillance, and efforts need to be made to ensure capacity for rapid diagnostic testing across sub-Saharan Africa, as well as the ability to rule out other tropical infections. In the above noted Ebola hemorrhagic fever case in Uganda in May 2011, in-country laboratory capacity was available, and a rapid diagnosis was made on the index case, allowing for an immediate public health response (MacNeil & Rollin, 2012).
In sub-Saharan African countries, there is a high burden of infectious disease and non-specific symptoms are commonly seen in many patients, especially those presenting to health clinics and hospitals with malaria and complications of HIV. This means that during a viral hemorrhagic fever outbreak there are many people coming to healthcare facilities near to the epicenter who may have similar symptoms to those with viral hemorrhagic fevers, but who are not exposed or infected (Parkes-Ratanshi et al., 2014).
When cases of the disease do appear, healthcare workers must be able to recognize a case of Ebola virus disease and be ready to employ isolation precautions and barrier-nursing techniques. They should also have the capability to request diagnostic tests or prepare samples for shipping and testing elsewhere.
Treatments Tried in Previous Ebola Outbreaks
When an Ebola outbreak occurs, healthcare providers, family members, townspeople, government officials, and traditional healers try everything at their disposal to treat infected patients and stop the spread of the virus.
In previous filovirus (Ebola, Marburg) outbreaks, in many cases, antibiotics were used to prevent or treat secondary bacterial infections. Analgesics, antipyretics, and antiemetic drugs were typically available and administered as needed. Unfortunately, many patients did not receive any further care. Other symptomatic treatments occasionally available included antidiarrheal drugs, sedatives, and antipsychotic drugs to reduce anxiety and agitation (Clark et al., 2012).
Oral rehydration was routinely encouraged, but at times not administered partially due to the close proximity required to prop up a severely ill patient so they can drink. Oral rehydration was typically preferred to administration of IV fluids, partially due to the perceived risk of transmission associated with the use of needles as well as resource constraints. Fluid and electrolyte monitoring and supplementation were universally applied to patients in well-equipped hospitals, but these measures were not routinely available during most outbreaks (Clark et al., 2012).
Various blood products, clotting factors, inhibitors of fibrinolysis, and regulators of coagulation were administered to counteract hemorrhage. Transfusion of blood components included whole blood, packed red blood cells, fresh frozen plasma, and platelets. Clotting factors and other regulators of coagulation administered included fibrinogen, and prothrombin, proconvertin, Stuart-factor and anti-hemophilic globulin B, and vitamin K. In contrast, anticoagulants (heparin) and rheologic agents (pentoxyfylline)* were given to some patients to prevent thrombosis and disseminated intravascular coagulation (Clark et al., 2012).
*Rheologic agents: Agents that alter blood viscosity, possibly improving blood flow.
None of the supportive care strategies used in the field during previous filovirus outbreaks have been prospectively evaluated to determine treatment efficacy. Transfusion of blood from convalescent patients* was highlighted as potentially useful in Kikwit, Zaire when only 1 of 8 patients receiving a transfusion died. However, these patients received substantially better care than those in the early stages of the epidemic. Ebola convalescent serum had been administered to three additional patients in two separate outbreaks, all of whom survived. Five patients in four separate outbreaks received IV heparin; 2 of the 5 patients survived. Dehydration was noted in several outbreaks as potentially contributing to the high mortality but, as with other therapies, the effect of IV fluid administration has not been rigorously evaluated (Clark et al., 2012).
Convalescent serum: Using blood from people who have recovered from an infection to treat patients still fight the infection.
Towards the end of the West Africa outbreak, trials for a new vaccine began, and by late 2016 had shown promising evidence that the vaccine was both safe and effective against the Zaire strain of Ebola virus. This vaccine, known as rVSV-ZEBOV, is now being used in responding to the ongoing Ebola outbreak in Democratic Republic of Congo. But for several reasons, including not having official licensure for the vaccine, it must be used under very restrictive conditions that severely limit the speed, and therefore the reach, of vaccination efforts (MSF, 2020).
