The immune system develops a defense against foreign substances. This defense is known as the immune response and usually involves the production of protein molecules (immunoglobulins or antibodies) by B-lymphocytes (B-cells) and specific cells, including T-lymphocytes (Pink Book, 2020, June 29).
Immunity is the ability of the human body to tolerate substances indigenous to the body and to eliminate foreign substances. Immunity is generally specific to a single organism or group of closely related organisms. The ability to eliminate foreign substances lies in the immune system. Since most organisms are identified as foreign, the ability to identify and eliminate these substances provides protection from infectious disease (Pink Book, 2020, June 29).
Vaccines
Vaccination is one of the world’s most important medical interventions. Generally safe, effective, and relatively inexpensive, vaccines save about three million lives every year and protect hundreds of millions of people against acute and chronic infections and their consequences. While administering a vaccine is a fairly simple process, the enterprise of vaccination is complex. To invent, test, and produce a vaccine is difficult, and protecting a population of people against infectious diseases requires high levels of organization and participation (Sabin Institute, 2020).
Vaccines stimulate the immune system to produce immune responses that protect against infection. Vaccines provide a safe, cost-effective, and efficient means of preventing illness, disability, and death from infectious diseases. There is more than one type of vaccine, although each is designed to teach the immune system how to fight off certain kinds of pathogens—and the serious diseases they cause. Types of vaccines include:
- Live-attenuated vaccines
- Inactivated vaccines
- Subunit, recombinant, polysaccharide, and conjugate vaccines
- Nucleic acid vaccines
Live-attenuated and Inactivated Vaccines
[Unless otherwise noted, the information in the following sections is taken from NIAID, Vaccine Types, July 1, 2019]
Traditional vaccines consist of entire pathogens that have been killed or weakened so that they cannot cause disease. Such whole-pathogen vaccines can elicit strong protective immune responses. Many of the vaccines in clinical use today fall into this category. However, not every disease-causing microbe can be effectively targeted with a whole-pathogen vaccine.
Measles Particle
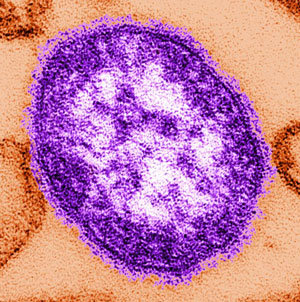
In 1954 Thomas Peebles and John Enders collected blood from students with measles at a private school near Boston. The measles virus was isolated and used to create a series of vaccines. The picture shows a thin-section transmission electron microscopic image of a single measles virus particle, with the viral nucleocapsid situated underneath the viral envelope, surrounded by surface projections. Source: Courtesy of CDC/Cynthia S. Goldsmith; and William Bellini, Ph.D. Used by permission.
Advances in tissue culture techniques have enabled development of live-attenuated vaccines, which contain a version of the living microbe that has been weakened in the laboratory. These vaccines elicit strong immune responses and can confer life-long immunity after only one or two doses. Live-attenuated vaccines are relatively easy to create for certain viruses, but difficult to produce for more complex pathogens like bacteria and parasites. Live vaccines are used to protect against:
- Measles, mumps, rubella (MMR combined vaccine)
- Rotavirus
- Smallpox
- Chickenpox
- Yellow fever
Inactivated, or killed, microbes have the ability to induce immunity. These inactivated vaccines are produced by killing the pathogen with chemicals, heat, or radiation. Inactivated vaccines usually do not provide immunity that is as strong as live vaccines. Several doses over time (booster shots) may be needed to get ongoing immunity against diseases. Inactivated vaccines are used to protect against:
- Hepatitis A
- Flu (shot only)
- Polio (shot only)
- Rabies
Modern genetic engineering techniques have enabled creation of chimeric viruses, which contain genetic information from, and display biologic properties of, different parent viruses. A NIAID-developed live-attenuated chimeric vaccine consisting of a dengue virus backbone with Zika virus surface proteins is undergoing early-stage testing in humans.
Subunit Recombinant, Polysaccharide, and Conjugate Vaccines
Subunit vaccines include only the components, or antigens, that best stimulate the immune system. Although this design can make vaccines safer and easier to produce, it often requires the incorporation of adjuvants* to elicit a strong protective immune response because the antigens alone are not sufficient to induce adequate long-term immunity.
*Adjuvants: substances formulated as part of a vaccine to boost immune responses and enhance the vaccine’s effectiveness.
Including only the essential antigens in a vaccine can minimize side effects, as illustrated by the development of a new generation of pertussis vaccines. The first pertussis vaccines, introduced in the 1940s, contained inactivated Bordetella pertussis bacteria. Although effective, whole-cell pertussis vaccines frequently caused minor adverse reactions such as fever and swelling at the injection site. This caused many people to avoid the vaccine, and by the 1970s decreasing vaccination rates had brought about an increase in new infections. Research led to the development of acellular pertussis vaccines that are based on individual, purified B. pertussis components. These vaccines are similarly effective to whole-cell vaccines but much less likely to cause adverse reactions.
Some vaccines to prevent bacterial infections are based on the polysaccharides, or sugars, that form the outer coating of many bacteria. The first licensed vaccine against Haemophilus influenzae type B (Hib) was a polysaccharide vaccine. However, its usefulness was limited because it did not elicit strong immune responses in infants—the age group with the highest incidence of Hib disease. Researchers then developed a so-called conjugate vaccine in which the Hib polysaccharide is attached, or conjugated, to a protein antigen to offer improved protection. This formulation greatly increased the ability of the immune systems of young children to recognize the polysaccharide and develop immunity.
Other vaccines against bacterial illnesses, such as diphtheria and tetanus vaccines, elicit immune responses against toxins (disease-causing proteins) secreted by the bacteria. The antigens in these vaccines are chemically inactivated toxins, known as toxoids.
In the 1980s, recombinant DNA technology—which enables DNA from two or more sources to be combined—was harnessed to develop the first recombinant protein vaccine, the hepatitis B vaccine. The vaccine antigen is a hepatitis B virus protein produced by yeast cells into which the genetic code for the viral protein has been inserted.
Vaccines to prevent human papillomavirus (HPV) infection also are based on recombinant protein antigens. In the early 1990s, scientists discovered that proteins from the outer shell of HPV can form particles that closely resemble the virus. These virus-like particles prompt an immune response similar to that elicited by the natural virus, but the virus-like particles are non-infectious because they do not contain the genetic material the virus needs to replicate inside cells.
Scientists are also developing new strategies to present protein subunit antigens to the immune system. As part of efforts to develop a universal flu vaccine, NIAID scientists designed an experimental vaccine featuring the protein ferritin, which can self-assemble into microscopic pieces called nanoparticles that display a protein antigen. An experimental nanoparticle-based influenza vaccine is being evaluated in an early-stage trial in humans.
Other relatively recent advances in laboratory techniques, such as the ability to solve atomic structures of proteins, have contributed to advances in subunit vaccine development. For example, by solving the three-dimensional structure of a protein on the RSV surface bound to an antibody, NIAID scientists identified a key area of the protein that is highly sensitive to neutralizing antibodies. They were then able to modify the RSV protein to stabilize the structural form it displays in the neutralization-sensitive site.
Antibody Binding to Virus
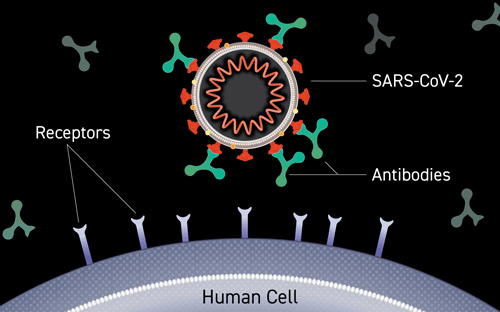
Illustration of an antibody binding to the surface of a virus, blocking entry into a person’s cells. Credit: Courtesy of Lisa Donohue, CoVPN, and COVID-19 Prevention Network. Used by permission.
While most subunit vaccines focus on a particular pathogen, scientists also are developing vaccines that could offer broad protection against various diseases. In 2017 scientists launched an early-phase clinical trial of a vaccine to prevent mosquito-borne diseases such as malaria, Zika, chikungunya, and dengue fever. The experimental vaccine, designed to trigger an immune response to mosquito saliva rather than a specific virus or parasite, contains four recombinant proteins from mosquito salivary glands.
Subunit, recombinant, polysaccharide, and conjugate vaccines are used to protect against:
- Hib (Haemophilus influenzae type b) disease
- Hepatitis B
- HPV (Human papillomavirus)
- Whooping cough (part of the DTaP combined vaccine)
- Pneumococcal disease
- Meningococcal disease
- Shingles
Nucleic Acid Vaccines
Another investigational approach to vaccination involves introducing genetic material encoding the antigen or antigens against which an immune response is sought. The body’s own cells then use this genetic material to produce the antigens. Potential advantages of this approach include the stimulation of broad long-term immune responses, excellent vaccine stability, and relative ease of large-scale vaccine manufacture.
DNA Vaccines
DNA plasmid vaccines comprise a small circular piece of DNA called a plasmid that carries genes encoding proteins from the pathogen of interest. The manufacturing process for DNA plasmid vaccines is well-established, allowing experimental vaccines to be quickly developed to address emerging or re-emerging infectious diseases. NIAID’s Vaccine Research Center has developed candidate DNA vaccines to address several viral disease threats during outbreaks, including SARS-CoV-1 in 2003, H5N1 avian influenza in 2005, H1N1 pandemic influenza in 2009, and Zika virus in 2016. The time from selection of the viral genes to be included in the vaccine to initiation of clinical studies in humans was shortened from 20 months with SARS-CoV-1 to slightly longer than 3 months with Zika virus.
Messenger RNA (mRNA) Vaccines
Vaccines based on messenger RNA (mRNA) are an intermediary between DNA and protein. mRNA vaccines teach our cells how to make a protein—or even just a piece of a protein—that triggers an immune response inside our bodies. That immune response, which produces antibodies, is what protects us from getting infected if the real virus enters our bodies. Recent advances have largely overcome issues with the instability of mRNA and the difficulty of delivering it into cells, and mRNA vaccines have demonstrated good efficacy against COVID-19.
mRNA vaccines have other key advantages over traditional vaccines or DNA-based vaccine. The first and foremost is safety. mRNA is noninfectious, so it will not be integrated into the recipient’s genome and it can be digested by normal cellular processes. In addition, the efficiency of mRNA delivery can be increased through designing and packaging the mRNA into protective carrier molecules, which enhance stability and encourage rapid uptake by the cells. mRNA vaccines are also scalable, as they rely on in vitro transcriptions (chemical reactions that are commonly practiced in laboratories) rather than on external factors such as the availability of hen’s eggs and the need for laboratory manipulation (Chin, 2020, July 20).
COVID-19 Vaccines
Clinical Considerations
Vaccination is the most effective way to prevent SARS-CoV-2 infection. NIH recommends COVID-19 vaccination as soon as possible for everyone who is eligible according to CDC’s Advisory Committee on Immunization Practices. Three vaccines are authorized or approved for use in the United States to prevent COVID-19. For primary and booster vaccinations, the mRNA vaccines (i.e., BNT162b2 [Pfizer-BioNTech] or mRNA-1273 [Moderna]) are preferable to the Ad26.COV2.S (Johnson & Johnson/Janssen) vaccine due to its risk of serious adverse events (NIH, 2022, May 13).
A primary series of COVID-19 vaccinations is recommended for everyone aged ≥5 years in the United States. Certain groups of people should receive additional doses at specified intervals after the primary series of vaccinations. The type and dose of vaccine and the timing of these additional doses depend on the recipient’s age and underlying medical conditions. CDC regularly updates the clinical considerations for use of the COVID-19 vaccines that are currently approved by the Food and Drug Administration (FDA) or authorized for use in the United States (NIH, 2022, May 13).
Vaccine Approval Timeline
On December 11, 2020, to great fanfare, the FDA issued the first emergency use authorization (EUA) for the Pfizer-BioNTech COVID-19 vaccine for individuals 16 years of age and older. High vaccine efficacy (95.0%) was reported in preventing COVID-19 following receipt of two doses of the vaccine.
Source: CDC
On December 18, 2020, an EUA was issued for the Moderna COVID-19 vaccine for use in individuals 18 years of age and older. High vaccine efficacy (94.1%) was reported in preventing COVID-19 following receipt of two doses of Moderna COVID-19 vaccine.
On May 10, 2021, the FDA issued an EUA for the Pfizer-BioNTech COVID-19 vaccine for use in adolescents ages 12-15 years. It has been found to be safe and effective in adolescents with side effects were generally consistent with those experienced by young adults. Side effects are typically normal signs that the body is building protection against the virus that causes COVID-19 (CDC, 2021, May 14).
On August 23, 2021, the FDA approved the first COVID-19 vaccine. The Pfizer-BioNTech COVID-19 vaccine (also called Comirnaty) was made available to individuals 16 years of age and older. The vaccine also continues to be available under an EUA for individuals 12 through 15 years of age and for the administration of a third dose in certain immunocompromised individuals.
On October 29, 2021, the FDA granted an emergency use authorization for the use of the Pfizer-BioNTech vaccine in children aged 5-11. The company continues to test the effectiveness of the vaccine for children aged 6 months to 2 years and 2 years to 5 years.
 CDC, public domain.
CDC, public domain.
An EUA of the Janssen (Johnson & Johnson) COVID-19 vaccine was granted February 27, 2021. The Janssen vaccine is a recombinant vector vaccine that uses a human adenovirus to express the spike protein found on the surface of the SARS-CoV-2 virus. The adenovirus vector has been modified so it cannot replicate in humans and cause illness. Use was paused in mid-April, 2021, after detection of six cases of cerebral venous sinus thrombosis. Shortly thereafter, the FDA amended the EUA to include information about this very rare and serious type of blood clot in people who receive the vaccine. The amended EUA allows the Janssen COVID-19 vaccine to be distributed in the U.S. for use in individuals 18 years of age and older.
The single-dose Janssen vaccine is 77% effective in preventing severe/critical COVID-19 occurring at least 14 days after vaccination and 85% effective in preventing severe/critical COVID-19 occurring at least 28 days after vaccination. The vaccine is approximately 67% effective in preventing moderate-to-severe/critical disease occurring at least 14 days after vaccination and 66% effective in preventing moderate-to-severe/critical disease occurring at least 28 days after vaccination. Importantly, the vaccine has been shown to be 100% effective in protecting against death from the disease everywhere it has been tested. The Janssen vaccine has the advantage of requiring only a single injection and can be easily transported and stored without special refrigeration requirements.
On December 16, 2021, the CDC endorsed recommendations from the Advisory Committee on Immunization Practices (ACIP), which expressed a clinical preference for individuals to receive an mRNA COVID-19 vaccine over the Johnson & Johnson vaccine. ACIP’s unanimous recommendation followed consideration of the latest evidence on vaccine effectiveness, vaccine safety and rare adverse events, and consideration of the U.S. vaccine supply. This CDC recommendation follows similar recommendations from other countries, including Canada and the United Kingdom (CDC, 2021, December 16).
Vaccine Adjuvants
[Unless otherwise noted, the following information is from NIAID, Vaccine Adjuvants, July 2, 2019.]
Efforts to develop safe and effective vaccines increasingly involve the use of adjuvants—substances formulated as part of a vaccine to boost immune responses and enhance the vaccine’s effectiveness. Adjuvants help the body to produce an immune response strong enough to protect people from the disease they are being vaccinated against. Adjuvanted vaccines can cause more local reactions (such as redness, swelling, and pain at the injection site) and more systemic reactions (such as fever, chills and body aches) than non-adjuvanted vaccines.
Vaccine adjuvants accelerate, enhance, and prolong the immune responses triggered by antigens—the vaccine components that elicit pathogen-specific immune responses. Certain populations, such as people with compromised immune systems, elders, and the very young particularly benefit from vaccines with adjuvants because their immune systems may require an extra boost to provide protection. Adjuvants can also allow vaccine developers to use less antigen, which in some cases may be in short supply, or costly. Moreover, adjuvanted vaccines can elicit more durable immune responses, reducing or eliminating the need for booster vaccinations.
Aluminum-containing adjuvants, collectively termed alum, have been safely used in vaccines since the 1930s and are still widely used today. Aluminum is among the most common metals found in nature and is present in food and water. Scientific research has shown that the trace amounts of aluminum in vaccines are safe and not readily absorbed by the body.
For many years, alum was the only adjuvant added to vaccines in the United States. In recent decades, however, scientific advances have increased our understanding of human immunity, and these insights have led to the identification of new adjuvants and promising adjuvant candidates. In 2009 FDA-approved Cervarix, a human papillomavirus vaccine containing a novel adjuvant called AS04. Since then, several additional vaccines containing novel adjuvants have been approved for use in the United States.
Learning more about how adjuvants work to stimulate specific immune responses is critical to the development of new and improved vaccines. Adjuvant research lays a foundation for vaccine developers to improve the protection that current vaccines offer, design vaccines for emerging infectious diseases, expedite efforts to develop vaccines to protect against diseases without preventive inoculations (e.g., HIV, tuberculosis), and develop vaccines to treat allergies, autoimmune diseases, and cancer.
What Happens If We Stop Vaccinating?
Before the middle of the last century, diseases like whooping cough, polio, measles, flu, and rubella struck hundreds of thousands of infants, children, and adults in the United States. Thousands died every year from them. As vaccines were developed and became widely used, rates of these diseases declined, until today most of them are nearly gone from our country (CDC, 2018, June 29).
- Before there was a vaccine, nearly everyone in the United States got measles, and hundreds died from it each year. Today, most doctors have never seen a case of measles.
- More than 15,000 Americans died from diphtheria in 1921, before there was a vaccine. Only 2 cases of diphtheria have been reported to CDC between 2004 and 2014.
- An epidemic of rubella (German measles) in 1964–1965 infected 12½ million Americans, killed 2,000 babies, and caused 11,000 miscarriages. Since 2012, 15 cases of rubella were reported to CDC. (CDC, 2018, June 29)
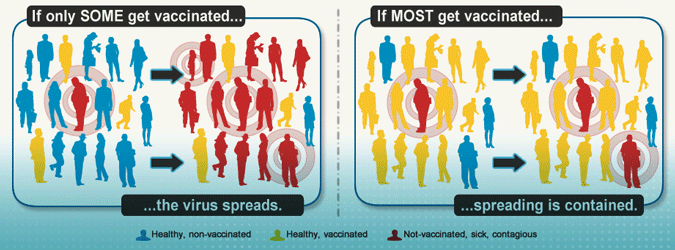
If one or two cases of disease are introduced into a community where most people are not vaccinated, outbreaks will occur. In 2013, for example, several measles outbreaks occurred around the country, including large outbreaks in New York City and Texas—mainly among groups with low vaccination rates. If vaccination rates dropped to low levels nationally, diseases could become as common as they were before vaccines. Source: CDC, 2018.
Anti-Viral, Immune-based, and Adjunctive Therapies
The COVID-19 pandemic has led to a global struggle to cope with the sheer numbers of infected people, many of whom require intensive care. The outbreak has been managed by a combination of public health measures, supportive care, and vaccination. The urgency to identify supportive treatments has led to the emergence of several investigational drugs as potential candidates to improve outcomes, especially in the severe to critically ill. While many of these drugs are being investigated in clinical trials, professional groups have attempted to clarify situations where the use of these drugs may be considered as off-label or compassionate (Xu et al., 2020).
Antiviral Drugs
Antiviral therapies inhibit viral entry, viral membrane fusion, and endocytosis.* Because viral replication may be particularly active early in the course of COVID-19, antiviral therapy may have the greatest impact before the illness progresses into the hyperinflammatory state seen in later stages of the disease. For this reason, understanding the role of antivirals in treating mild, moderate, severe, and critical illness is necessary to optimize treatment for people with COVID-19 (NIAID-RML, 2020, July 30).
*Endocytosis: a cellular process by which substances are brought into the cell. It is a type of active transport that moves particles, such as large molecules, parts of cells, and even whole cells, into a cell.
Remdesivir is the only drug that is approved by the Food and Drug Administration for the treatment of COVID-19. Ritonavir-boosted nirmatrelvir (Paxlovid), molnupiravir, and certain anti-SARS-CoV-2 monoclonal antibodies (mAbs) have received Emergency Use Authorizations from the FDA for the treatment of COVID-19 (NIH, 2022, May 13).
NIH recommends against the use of the following drugs for the treatment of COVID-19, except in a clinical trial:
- Interferons for nonhospitalized patients
- Interferon alfa or lambda for hospitalized patients
- Ivermectin
- Nitazoxanide (NIH, 2022, May 13)
The NIH recommends against the use of the following drugs for the treatment of COVID-19:
- Chloroquine or hydroxychloroquine and/or azithromycin for hospitalized (AI) and nonhospitalized patients
- Lopinavir/ritonavir and other HIV protease inhibitors for hospitalized (AI) and nonhospitalized patients
- Systemic interferon beta for hospitalized patients (NIH, 2022, May 13)
Immune-based Therapies
Given the hyperactive inflammatory effects of SARS-CoV-2, agents that modulate the immune response are being explored as adjunctive treatments for the management of moderate to critical COVID-19. These agents include human blood–derived products and immunomodulatory therapies (NIAID-RML, 2020, July 30).
Some human blood–derived products are obtained from individuals who have recovered from SARS-CoV-2 infection (e.g., convalescent plasma, immunoglobulin products). These products are thought to have either direct antiviral properties, such as in convalescent plasma, and/or immunomodulatory effects. Additionally, neutralizing monoclonal antibodies directed against SARS-CoV-2 have been developed and are under investigation in clinical trials (NIAID-RML, 2020, July 30).
Other agents in this group include therapeutics currently approved for the treatment of other immune and/or inflammatory syndromes. These agents include corticosteroids (e.g., glucocorticoids), which as a class possess a broad array of mechanisms to treat systemic inflammation, and more targeted anti-inflammatory treatments such as interleukin inhibitors, interferons, kinase inhibitors, and others (NIAID-RML, 2020, July 30).
Adjunctive Therapies
Antithrombotic Therapy. Infection with SARS-CoV-2 has been associated with inflammation and a prothrombotic state, with increases in fibrin, fibrin degradation products, fibrinogen, and D-dimers. In fact, these markers have been associated with worsened clinical outcomes. Although the true incidence of these complications among those with differing severities of disease is not completely defined, there have been reports of increased incidence of thromboembolic disease associated with COVID-19 in patients in the intensive care unit.
For a summary of the use of antithrombotic therapy in patients with COVID-19, please go here.
Vitamin C. Vitamin C (ascorbic acid) is a water-soluble vitamin that is thought to have beneficial effects in patients with severe and critical illnesses. It is an antioxidant and free radical scavenger that has anti-inflammatory properties, influences cellular immunity and vascular integrity, and serves as a cofactor in the generation of endogenous catecholamines (NIAID-RML, 2020, July 30).
Because humans may require more vitamin C in states of oxidative stress, vitamin C supplementation has been evaluated in numerous disease states, including serious infections and sepsis. Because serious COVID-19 may cause sepsis and acute respiratory distress syndrome (ARDS),researchers are studying the potential role of high doses of vitamin C in ameliorating inflammation and vascular injury in patients with COVID-19. Nevertheless, insufficient data are available for the COVID-19 Treatment Guidelines panel to recommend either for or against the use of vitamin C for treatment of COVID-19 in noncritically ill patients (NIAID-RML, 2020, July 30).
Vitamin D. Vitamin D is critical for bone and mineral metabolism. Because the vitamin D receptor is expressed on immune cells such as B cells, T cells, and antigen-presenting cells, and because these cells can synthesize the active vitamin D metabolite, vitamin D also has the potential to modulate innate and adaptive immune responses (NIAID-RML, 2020, July 30).
Vitamin D deficiency is common in the United States, particularly among Black persons or those of Hispanic ethnicity. Vitamin D deficiency is also more common in older patients and patients with obesity and hypertension; these factors have been associated with worsened outcomes in patients with COVID-19. In observational studies, low vitamin D levels have been associated with an increased risk of community-acquired pneumonia in older adults and children. Vitamin D supplements may increase the levels of T regulatory cells in healthy individuals and patients with autoimmune diseases; vitamin D supplements may also increase T regulatory cell activity (NIAID-RML, 2020, July 30).
In a meta-analysis of randomized clinical trials, vitamin D supplementation was shown to protect against acute respiratory tract infection. However, in two randomized, double-blind, placebo-controlled clinical trials, administering high doses of vitamin D to critically ill patients with vitamin D deficiency (but not COVID-19) did not reduce the length of the hospital stay or the mortality rate when compared to placebo. High levels of vitamin D may cause hypercalcemia and nephrocalcinosis. Overall, insufficient data exist to recommend either for or against the use of vitamin D for the prevention or treatment of COVID-19 (NIAID-RML, 2020, July 30).
Zinc Supplementation. The COVID-19 Treatment Guidelines panel recommends against using zinc supplementation above the recommended dietary allowance for the prevention of COVID-19, except in a clinical trial.
Increased intracellular zinc concentrations impairs replication in a number of RNA viruses. Zinc has been shown to enhance cytotoxicity and induce apoptosis* when used in vitro with a zinc ionophore such as chloroquine. Chloroquine has also been shown to enhance intracellular zinc uptake in vitro. The relationship between zinc and COVID-19, including how zinc deficiency affects the severity of COVID-19 and whether zinc supplements can improve clinical outcomes, is currently under investigation (NIAID-RML, 202, July 30).
*Apoptosis: Apoptosis is the process of programmed cell death. It is a process that rids the body of cells that have been damaged beyond repair. Apoptosis also plays a role in preventing cancer.
Corticosteroids
Patients with severe COVID-19 can develop a systemic inflammatory response that can lead to lung injury and multisystem organ dysfunction. It has been proposed that the potent anti-inflammatory effects of corticosteroids might prevent or mitigate these deleterious effects. The Randomised Evaluation of COVID-19 Therapy (RECOVERY) trial showed that the mortality rate was lower among patients who were randomized to receive dexamethasone than among those who received the standard of care. This benefit was observed in patients who required supplemental oxygen at enrollment. No benefit of dexamethasone was seen in patients who did not require supplemental oxygen at enrollment (NIAID-RML, 2020, August 27).
Both beneficial and deleterious clinical outcomes have been reported with use of corticosteroids (mostly prednisone or methylprednisolone) in patients with other pulmonary infections. In patients with Pneumocystis jirovecii pneumonia and hypoxia, prednisone therapy reduced the risk of death; however, in outbreaks of other novel coronavirus infections (i.e., Middle East respiratory syndrome [MERS] and severe acute respiratory syndrome [SARS]), corticosteroid therapy was associated with delayed virus clearance. In severe pneumonia caused by influenza viruses, corticosteroid therapy appears to result in worse clinical outcomes, including secondary bacterial infection and death (NIAID-RML, 2020, August 27).
On July 30, 2020 the recommendations were updated to allow the use of alternative corticosteroids (i.e., hydrocortisone, methylprednisolone, prednisone) in situations where dexamethasone may not be available (NIAID-RML, 2020, July 30).
Chloroquine or Hydroxychloroquine
Chloroquine is an antimalarial drug that was developed in 1934. Hydroxychloroquine, an analogue of chloroquine, was developed in 1946 and is used to treat autoimmune diseases [e.g., systemic lupus erythematosus (SLE), rheumatoid arthritis]. In general, hydroxychloroquine has fewer and less severe toxicities and fewer drug–drug interactions than chloroquine (NIAID-RML, 2020 July 30).
High-dose chloroquine has been associated with more severe toxicities than lower-dose chloroquine. A comparative trial compared high-dose chloroquine and low-dose chloroquine in patients with COVID-19; in addition, all participants received azithromycin, and 89% of the participants received oseltamivir. The study was discontinued early when preliminary results showed higher rates of mortality and QTc prolongation in the high-dose chloroquine group (NIAID-RML, 2020 July 30).
The COVID-19 Treatment Guidelines recommend against the use of chloroquine or hydroxychloroquine for the treatment of COVID-19, except in a clinical trial. The panel recommends against the use of high-dose chloroquine for the treatment of COVID-19 (NIAID-RML, 2020, July 30).
